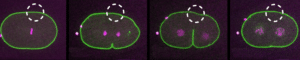
Ablation & Microdissection
2024
- Mangeol, Pierre, Dominique Massey-Harroche, Michael Sebbagh, Fabrice Richard, André Le Bivic, and Pierre-François Lenne. 2024. “The Zonula Adherens Matura Redefines the Apical Junction of Intestinal Epithelia.” Proceedings of the National Academy of Sciences 121(9):2017. doi: 10.1073/pnas.2316722121.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn)
2023
- Bell-Simons, M., S. Buchholz, J. Klimek, and H. Zempel. 2023 “Laser-Induced Axotomy of Human IPSC-Derived and Murine Primary Neurons Decreases Somatic Tau and AT8 Tau Phosphorylation: A Single-Cell Approach to Study Effects of Acute Axonal Damage.” Cellular and Molecular Neurobiology 43(7):3497–3510. doi: 10.1007/s10571-023-01359-z.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Chandran, Lenin, Wilko Backer, Raphael Schleutker, Deqing Kong, Seyed A. H. Beati, Stefan Luschnig, and H. Arno J. Müller. 2023 “Src42A Is Required for E-Cadherin Dynamics at Cell Junctions during Drosophila Axis Elongation.” Development 150(2):37–39. doi: 10.1242/dev.201119.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Cohen, Roie, Shahar Taiber, Olga Loza, Shahar Kasirer, Shiran Woland, and David Sprinzak. 2023. “Precise Alternating Cellular Pattern in the Inner Ear by Coordinated Hopping Intercalations and Delaminations.” Science Advances 9(8). doi: 10.1126/sciadv.add2157.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Esser, Lisann, Ronald Springer, Georg Dreissen, Lukas Lövenich, Jens Konrad, Nico Hampe, Rudolf Merkel, Bernd Hoffmann, and Erik Noetzel. 2023 “Elastomeric Pillar Cages Modulate Actomyosin Contractility of Epithelial Microtissues by Substrate Stiffness and Topography.” Cells 12(9). doi: 10.3390/cells12091256.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn). - Hirschhäuser, Alexander, Darius Molitor, Gabriela Salinas, Jörg Großhans, Katja Rust, and Sven Bogdan. 2023. “ Single-Cell Transcriptomics Identifies New Blood Cell Populations in Drosophila Released at the Onset of Metamorphosis .” Development. doi: 10.1242/dev.201767.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn. - Kennard, Andrew S., Mugdha Sathe, Ellen C. Labuz, Christopher K. Prinz, and Julie A. Theriot. 2023. “Post-Injury Hydraulic Fracturing Drives Fissure Formation in the Zebrafish Basal Epidermal Cell Layer.” Current Biology 33(13):2616-2631.e5. doi: 10.1016/j.cub.2023.05.021.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-405/120 - Peterman, Eric, Elgene J. A. Quitevis, Erik C. Black, Emma C. Horton, Rune L. Aelmore, Ethan White, Alvaro Sagasti, and Jeffrey P. Rasmussen. 2023. “Zebrafish Cutaneous Injury Models Reveal Langerhans Cells Engulf Axonal Debris in Adult Epidermis.” Disease Models & Mechanisms. doi: 10.1242/dmm.049911.
Equipment used: Photomanipulation system AiWon with pulsed 42mW 532nm laser DPSL-532/42 (UGA-42 Caliburn) - Schmidt, Anja, Tara Finegan, Matthias Häring, Deqing Kong, Alexander G. Fletcher, Zuhayr Alam, Jörg Grosshans, Fred Wolf, and Mark Peifer. 2023. “Polychaetoid/ZO-1 Strengthens Cell Junctions under Tension While Localizing Differently than Core Adherens Junction Proteins” edited by A. Yap. Molecular Biology of the Cell 1–23. doi: 10.1091/mbc.E23-03-0077.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 355nm laser Caliburn 355/42 (UGA-42 Caliburn).
2022
- Boscq, Samuel, Stéphanie Dutertre, Ioannis Theodorou, and Bénédicte Charrier. 2022. “Targeted Laser Ablation in the Embryo of Saccharina Latissima.” Journal of Visualized Experiments (181). doi: 10.3791/63518.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 25mW UV laser DPSL-355/25 (UGA-42 Caliburn) - Lay, Angelina J., Alexander Dupuy, Lejla Hagimola, Jessica Tieng, Mark Larance, Yunwei Zhang, Jean Yang, Yvonne Xiangyue Kong, Joyce Chiu, Emilia Charlotte Gray, Zihao Qin, Diana Schmidt, Jessica A. A. Maclean, Benjamin Rink Hofma, Marc L. Ellis, Maggie L. Kalev-Zylinska, Yair Argon, Shaun P. Jackson, Philip Hogg, and Freda H. Passam. 2022 “Endoplasmic Reticulum Protein 5 Attenuates Platelet Endoplasmic Reticulum Stress and Secretion in a Mouse Model.” Blood Advances. doi: 10.1182/bloodadvances.2022008457.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Lehne, Franziska, Thomas Pokrant, Sabnam Parbin, Gabriela Salinas, Jörg Großhans, Katja Rust, Jan Faix, and Sven Bogdan. 2022 “Calcium Bursts Allow Rapid Reorganization of EFhD2/Swip-1 Cross-Linked Actin Networks in Epithelial Wound Closure.” Nature Communications 13(1):1–17. doi: 10.1038/s41467-022-30167-0.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 355nm laser Caliburn 355/42 (UGA-42 Caliburn). - Lv, Zhiyi, Na Zhang, Xiaozhu Zhang, Jörg Großhans, and Deqing Kong. 2022. “The Lateral Epidermis Actively Counteracts Pulling by the Amnioserosa During Dorsal Closure.” Frontiers in Cell and Developmental Biology 10(May):1–15. doi: 10.3389/fcell.2022.865397.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) and diode single line laser DL-375 - Martin, Emmanuel, and Magali Suzanne. 2022 “MBeRFP: A Versatile Fluorescent Tool to Enhance Multichannel Live Imaging and Its Applications.” Development (Cambridge) 149(16). doi: 10.1242/dev.200495.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 532nm laser DPSS-532 (UGA-42 Caliburn). - Roellig, Daniela, Sophie Theis, Amsha Proag, Guillaume Allio, Bertrand Bénazéraf, Jérôme Gros, and Magali Suzanne. 2022 “Force-Generating Apoptotic Cells Orchestrate Avian Neural Tube Bending.” Developmental Cell 707–18. doi: 10.1016/j.devcel.2022.02.020.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 532nm laser DPSS-532 (UGA-42 Caliburn). - Sønder, Stine, Malene Ebstrup, Catarina Dias, Anne Sofie Heitmann, and Jesper Nylandsted. 2022 “Plasma Membrane Wounding and Repair Assays for Eukaryotic Cells.” BIO-PROTOCOL 12(11):1–18. doi: 10.21769/BioProtoc.4437.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 355nm laser Caliburn 355/42 (UGA-42 Caliburn).
2021
- Bastounis, Effie E., Francisco Serrano-Alcalde, Prathima Radhakrishnan, Patrik Engström, María J. Gómez-Benito, Mackenzi S. Oswald, Yi-Ting Yeh, Jason G. Smith, Matthew D. Welch, José M. García-Aznar, and Julie A. Theriot. 2021. “Mechanical Competition Triggered by Innate Immune Signaling Drives the Collective Extrusion of Bacterially Infected Epithelial Cells.” Developmental Cell 56(4):443-460.e11. doi: 10.1016/j.devcel.2021.01.012.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Bastounis, Effie E., Christopher K. Prinz, and Julie A. Theriot. “Protocol Volume Measurement and Biophysical Characterization of Mounds in Epithelial Monolayers after Intracellular Bacterial Infection Volume Measurement and Biophysical Characterization of Mounds in Epithelial Monolayers after Intracellular Bacterial In.” STAR Protocols 2(2):100551. doi: 10.1016/j.xpro.2021.100551.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Bischoff, Maik C., Sebastian Lieb, Renate Renkawitz-Pohl, and Sven Bogdan. 2021. “Filopodia-Based Contact Stimulation of Cell Migration Drives Tissue Morphogenesis.” Nature Communications 12(1):791. doi: 10.1038/s41467-020-20362-2.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Caroti, Francesca, Wim Thiels, Michiel Vanslambrouck, and Rob Jelier. 2021 “Wnt Signaling Induces Asymmetric Dynamics in the Actomyosin Cortex of the C. Elegans Endomesodermal Precursor Cell.” Frontiers in Cell and Developmental Biology 9(September):1–11. doi: 10.3389/fcell.2021.702741.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Dias, Catarina, Erisa Nita, Jakub Faktor, Ailish C. Tynan, Lenka Hernychova, Borivoj Vojtesek, Jesper Nylandsted, Ted R. Hupp, Tilo Kunath, and Kathryn L. Ball. 2021. “CHIP-Dependent Regulation of the Actin Cytoskeleton Is Linked to Neuronal Cell Membrane Integrity.” IScience 24(8):102878. doi: 10.1016/j.isci.2021.102878.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn). - Heitmann, Anne Sofie Busk, Ali Asghar Hakami Zanjani, Martin Berg Klenow, Anna Mularski, Stine Lauritzen Sønder, Frederik Wendelboe Lund, Theresa Louise Boye, Catarina Dias, Poul Martin Bendix, Adam Cohen Simonsen, Himanshu Khandelia, and Jesper Nylandsted. 2021. “Phenothiazines Alter Plasma Membrane Properties and Sensitize Cancer Cells to Injury by Inhibiting Annexin-Mediated Repair.” Journal of Biological Chemistry 101012. doi: 10.1016/j.jbc.2021.101012.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14 mW UV laser DPSL-355/14 (UGA-42 Caliburn). - Hirschhäuser, Alexander, Marianne van Cann, and Sven Bogdan. 2021 “CK1α Protects WAVE from Degradation to Regulate Cell Shape and Motility in Immune Response.” Journal of Cell Science 134(23). doi: 10.1242/jcs.258891
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Klenow, Martin Berg, Anne Sofie Busk Heitmann, Jesper Nylandsted, and Adam Cohen Simonsen. 2021. “Timescale of Hole Closure during Plasma Membrane Repair Estimated by Calcium Imaging and Numerical Modeling.” Scientific Reports 11(1):4226. doi: 10.1038/s41598-021-82926-6.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14 mW UV laser DPSL-355/14 (UGA-42 Caliburn). - Martin, Emmanuel, Sophie Theis, Guillaume Gay, Bruno Monier, Christian Rouvière, and Magali Suzanne. “Arp2/3-Dependent Mechanical Control of Morphogenetic Robustness in an Inherently Challenging Environment.” Developmental Cell 1–15. doi: 10.1016/j.devcel.2021.01.005.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 532nm laser DPSS-532 (UGA-42 Caliburn). - Mularski, Anna, Stine Lauritzen Sønder, Anne Sofie Busk Heitmann, Jesper Nylandsted, and Adam Cohen Simonsen. “Simultaneous Membrane Binding of Annexin A4 and A5 Suppresses 2D Lattice Formation While Maintaining Curvature Induction.” Journal of Colloid and Interface Science. doi: 10.1016/j.jcis.2021.05.067.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn). - Sinner, Marina P., Florentin Masurat, Jonathan J. Ewbank, Nathalie Pujol, and Henrik Bringmann. 2021 “Innate Immunity Promotes Sleep through Epidermal Antimicrobial Peptides.” Current Biology 31(3):564-577.e12. doi: 10.1016/j.cub.2020.10.076.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Sønder, Stine Lauritzen, Swantje Christin Häger, Anne Sofie Busk Heitmann, Lisa B. Frankel, Catarina Dias, Adam Cohen Simonsen, and Jesper Nylandsted. 2021. “Restructuring of the Plasma Membrane upon Damage by LC3-Associated Macropinocytosis.” Science Advances 7(27):eabg1969. doi: 10.1126/sciadv.abg1969.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn).
2020
- Abreu, Carla M., Luca Gaperini, Manuela E. L. Lago, Rui L. Reis, and Alexandra P. Marques. “Microscopy‐guided Laser Ablation for the Creation of Complex Skin Models with Folliculoid Appendages.” Bioengineering & Translational Medicine.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn). - Cohen, Roie, Liat Amir-Zilberstein, Micha Hersch, Shiran Woland, Olga Loza, Shahar Taiber, Fumio Matsuzaki, Sven Bergmann, Karen B. Avraham, and David Sprinzak. 2020. “Mechanical Forces Drive Ordered Patterning of Hair Cells in the Mammalian Inner Ear.” Nature Communications 11(1):5137.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Kakanj, Parisa, Sabine A. Eming, Linda Partridge, and Maria Leptin. 2020. “Long-Term in Vivo Imaging of Drosophila Larvae.” Nature Protocols 15(3):1158–87.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Nieuwenhuis, Bart, Amanda C. Barber, Rachel S. Evans, Craig S. Pearson, Joachim Fuchs, Amy R. MacQueen, Susan Erp, Barbara Haenzi, Lianne A. Hulshof, Andrew Osborne, Raquel Conceicao, Tasneem Z. Khatib, Sarita S. Deshpande, Joshua Cave, Charles Ffrench‐Constant, Patrice D. Smith, Klaus Okkenhaug, Britta J. Eickholt, Keith R. Martin, James W. Fawcett, and Richard Eva. 2020. “PI 3‐kinase Delta Enhances Axonal PIP 3 to Support Axon Regeneration in the Adult CNS.” EMBO Molecular Medicine 12(8):1–24.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Petrova, Veselina, Craig S. Pearson, Jared Ching, James R. Tribble, Andrea G. Solano, Yunfei Yang, Fiona M. Love, Robert J. Watt, Andrew Osborne, Evan Reid, Pete A. Williams, Keith R. Martin, Herbert M. Geller, Richard Eva, and James W. Fawcett. 2020 “Protrudin Functions from the Endoplasmic Reticulum to Support Axon Regeneration in the Adult CNS.” Nature Communications 11(1):5614.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Saadaoui, Mehdi, Didier Rocancourt, Julian Roussel, Francis Corson, and Jerome Gros. “A Tensile Ring Drives Tissue Flows to Shape the Gastrulating Amniote Embryo.” Science 367(6476):453–58. doi: 10.1126/science.aaw1965.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn). - Sridhar, Krishna Chander, Nils Hersch, Georg Dreissen, Rudolf Merkel, and Bernd Hoffmann. 2020 “Calcium Mediated Functional Interplay between Myocardial Cells upon Laser-Induced Single-Cell Injury: An in Vitro Study of Cardiac Cell Death Signaling Mechanisms.” Cell Communication and Signaling 18(1):1–14.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn).
2019
- Ambrosini, Arnaud, Mégane Rayer, Bruno Monier, and Magali Suzanne. 2019. “Mechanical Function of the Nucleus in Force Generation during Epithelial Morphogenesis.” Developmental Cell 50(2):197-211.e5.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-532/42 (UGA-42 Caliburn) - Balaji, Ramya, Vanessa Weichselberger, and Anne-Kathrin Classen. 2019. “Response of Drosophila Epithelial Cell and Tissue Shape to External Forces in Vivo.” Development 146(17):dev171256.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Gracia, Mélanie, Sophie Theis, Amsha Proag, Guillaume Gay, Corinne Benassayag, and Magali Suzanne. 2019. “Mechanical Impact of Epithelial−mesenchymal Transition on Epithelial Morphogenesis in Drosophila.” Nature Communications 10(1):1–17.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSS-532/42 (UGA-42 Caliburn) - Kong, Weiyuan, Olivier Loison, Pruthvi Chavadimane Shivakumar, Eunice Ho Yee Chan, Mehdi Saadaoui, Claudio Collinet, Pierre François Lenne, and Raphaël Clément. 2019. “Experimental Validation of Force Inference in Epithelia from Cell to Tissue Scale.” Scientific Reports 9(1):1–12.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-355/42 (UGA-42 Caliburn) - Lv, Z., Rosenbaum, J., Mohr, S., Zhang, X., Kong, D., Preiß, H., Kruss, S., Alim, K., Aspelmeier, T., Großhans, J. (2019). The emergent Yo-yo movement of nuclei driven by collective cytoskeletal remodeling in pseudo-synchronous mitotic cycles. BioRxiv. https://doi.org/https://doi.org/10.1101/662965
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Proag, Amsha, Bruno Monier, and Magali Suzanne. 2019. “Physical and Functional Cell-Matrix Uncoupling in a Developing Tissue under Tension.” Development 146(11):dev172577.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSS-532/42 (UGA-42 Caliburn) - Sønder, Stine Lauritzen, Theresa Louise Boye, Regine Tölle, Jörn Dengjel, Kenji Maeda, Marja Jäättelä, Adam Cohen Simonsen, Jyoti K. Jaiswal, and Jesper Nylandsted. 2019. “Annexin A7 Is Required for ESCRT III-Mediated Plasma Membrane Repair.” Scientific Reports 9(1):6726.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 42mW UV laser DPSL-532/42 (UGA-42 Caliburn)
2018
- Beati, Hamze, Irina Peek, Paulina Hordowska, Mona Honemann-Capito, Jade Glashauser, Fabian A. Renschler, Parisa Kakanj, Andreas Ramrath, Maria Leptin, Stefan Luschnig, Silke Wiesner, and Andreas Wodarz. 2018. “The Adherens Junction-Associated LIM Domain Protein Smallish Regulates Epithelial Morphogenesis.” Journal of Cell Biology 217(3):1079–95. Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
- Moreira, Carolina G. A., Jennifer C. Regan, Anna Zaidman-Rémy, Antonio Jacinto, and Soren Prag. 2018. Cell Migration. Vol. 1749. edited by A. Gautreau. New York, NY: Springer New York. Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
- Oranth, Alexandra, Christian Schultheis, Oleg Tolstenkov, Karen Erbguth, Jatin Nagpal, David Hain, Martin Brauner, Sebastian Wabnig, Wagner Steuer Costa, Rebecca D. McWhirter, Sven Zels, Sierra Palumbos, David M. Miller III, Isabel Beets, and Alexander Gottschalk. 2018. “Food Sensation Modulates Locomotion by Dopamine and Neuropeptide Signaling in a Distributed Neuronal Network.” Neuron 100(6):1414-1428.e10.
Equipment used: Illumination system KSL-2 with 473nm (KSL-2) - Otsuka, Shotaro, Anna M. Steyer, Martin Schorb, Jean Karim Hériché, M. Julius Hossain, Suruchi Sethi, Moritz Kueblbeck, Yannick Schwab, Martin Beck, and Jan Ellenberg. 2018. “Postmitotic Nuclear Pore Assembly Proceeds by Radial Dilation of Small Membrane Openings.” Nature Structural and Molecular Biology.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Prystopiuk, V., Fels, B., Simon, C. S., Liashkovich, I., Pasrednik, D., Kronlage, C., Wedlich-Söldner, R., Oberleithner, H., Fels, J. (2018). A two-phase response of endothelial cells to hydrostatic pressure. Journal of Cell Science. https://doi.org/10.1242/jcs206920
Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Rüder, Marike, Benedikt M. Nagel, and Sven Bogdan. 2018. “Analysis of Cell Shape and Cell Migration of Drosophila Macrophages in Vivo.” Pp. 227–38 in Methods in Molecular Biology. Vol. 1749. Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
2017
- Eva, R., Koseki, H., Kanamarlapudi, V., & Fawcett, J. W. (2017). EFA6 regulates selective polarised transport and axon regeneration from the axon initial segment. Journal of Cell Science, 130(21), 3663–3675. https://doi.org/10.1242/jcs.207423
Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Gayathri Vegesna, N. V., Ronchi, P., Durdu, S., Terjung, S., & Pepperkok, R. (2017). Targeted Ablation Using Laser Nanosurgery. https://doi.org/10.1007/978-1-4939-6810-7_8
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355 (UGA-42 Caliburn) - Karsch, Susanne, Deqing Kong, Jörg Großhans, and Andreas Janshoff. 2017. “Single-Cell Defects Cause a Long-Range Mechanical Response in a Confluent Epithelial Cell Layer.” Biophysical Journal 113(12):2601–8. https://doi.org/10.1016/j.bpj.2017.10.025
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Kilaru, S., Schuster, M., Ma, W., & Steinberg, G. (2017). Fluorescent markers of various organelles in the wheat pathogen Zymoseptoria tritici. Fungal Genetics and Biology, 105(March), 16–27. https://doi.org/10.1016/j.fgb.2017.05.001
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Caliburn) - Koseki, H., Donegá, M., Lam, B. Y., Petrova, V., van Erp, S., Yeo, G. S., Kwok, J.C.F., Constant, C., Eva, R., Fawcett, J. W. (2017). Selective rab11 transport and the intrinsic regenerative ability of CNS axons. ELife, 6, 1–25. https://doi.org/10.7554/eLife.26956
Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Prahlad, A., Spalthoff, C., Kong, D., Großhans, J., Göpfert, M. C., & Schmidt, C. F. (2017). Mechanical Properties of a Drosophila Larval Chordotonal Organ. Biophysical Journal, 113(12), 2796–2804. https://doi.org/10.1016/j.bpj.2017.08.061
Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Schwarz, J., & Bringmann, H. (2017). Analysis of the NK2 homeobox gene ceh-24 reveals sublateral motor neuron control of left-right turning during sleep. ELife, 6, 1–25. https://doi.org/10.7554/eLife.24846
Equipment used: Photomanipulation system AiWon with pulsed 14mW UV laser DPSL-355/14 (AiWon Caliburn) - Steinberg, G., Schuster, M., Hacker, C., Kilaru, S., & Correia, A. (2017). ATP prevents Woronin bodies from sealing septal pores in unwounded cells of the fungus Zymoseptoria tritici. Cellular Microbiology, 19(11), 1–10. https://doi.org/10.1111/cmi.12764
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Caliburn)
2016
- Brinkmann, Klaus, Moritz Winterhoff, Susanne Filiz Önel, Jörg Schultz, Jan Faix, and Sven Bogdan. 2016. “WHAMY Is a Novel Actin Polymerase Promoting Myoblast Fusion, Macrophage Cell Motility and Sensory Organ Development in Drosophila.” Journal of Cell Science 129(3):604–20.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Firmino, Joao, Didier Rocancourt, Mehdi Saadaoui, Chloe Moreau, and Jerome Gros. 2016. “Cell Division Drives Epithelial Cell Rearrangements during Gastrulation in Chick.” Developmental Cell 36(3):249–61.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Kakanj, Parisa, Bernard Moussian, Sebastian Grönke, Victor Bustos, Sabine A. Eming, Linda Partridge, and Maria Leptin. 2016. “Insulin and TOR Signal in Parallel through FOXO and S6K to Promote Epithelial Wound Healing.” Nature Communications 7.
Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
2014
- Lammel, U., M. Bechtold, I. Bunse, S. Bogdan, X. Jiang, A. Fleige, C. Klambt, D. Berh, and B. Risse. 2014. “The Drosophila FHOD1-like Formin Knittrig Acts through Rok to Promote Stress Fiber Formation and Directed Macrophage Migration during the Cellular Immune Response.” Journal of Cell Science 127(6):e1–e1. Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
2013
- Moreira, C. G. A., A. Jacinto, and S. Prag. 2013. “Drosophila Integrin Adhesion Complexes Are Essential for Hemocyte Migration in Vivo.” Biology Open 2(8):795–801. Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (=> UGA-42 Caliburn)
- Sander, Moritz, Anna Julia Squarr, Benjamin Risse, Xiaoyi Jiang, and Sven Bogdan. 2013. “Drosophila Pupal Macrophages – A Versatile Tool for Combined Ex Vivo and in Vivo Imaging of Actin Dynamics at High Resolution.” European Journal of Cell Biology 92(10–11):349–54. Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
- Wenzl, Christian, Philip Laupsien, Shuling Yan, Jörg Großhans, and Sreemukta Acharya. 2013. “Function and Dynamics of Slam in Furrow Formation in Early Drosophila Embryo.” Developmental Biology 386(2):371–84. Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
2012
- Ronchi, Paolo, Stefan Terjung, and Rainer Pepperkok. 2012. “At the Cutting Edge: Applications and Perspectives of Laser Nanosurgery in Cell Biology.” Pp. 235–48 in Biological Chemistry. Vol. 393. Equipment used: Photomanipulation system UGA-40 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
DNA Damage
2024
- Zhou, Di, Qing Yu, Roel C. Janssens, and Jurgen A. Marteijn. 2024. “Live-Cell Imaging of Endogenous CSB-MScarletI as a Sensitive Marker for DNA-Damage-Induced Transcription Stress.” Cell Reports Methods doi: 10.1016/j.crmeth.2023.100674.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266 (UGA-42 Caliburn)
2023
- Claessens, Laura A., Matty Verlaan-de Vries, Ilona J. de Graaf, and Alfred C. O. Vertegaal. 2023 “SENP6 Regulates Localization and Nuclear Condensation of DNA Damage Response Proteins by Group DeSUMOylation.” Nature Communications 14(1). doi: 10.1038/s41467-023-41623-w.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL 355/14 (UGA-42 Caliburn) - Pappas, George, Sebastian Howen Nesgaard Munk, Kenji Watanabe, Quentin Thomas, Zita Gál, Helena Hagner Gram, MyungHee Lee, Daniel Gómez-Cabello, Dimitris Christos Kanellis, Pedro Olivares-Chauvet, Dorthe Helena Larsen, Lea Haarup Gregersen, Apolinar Maya-Mendoza, Panagiotis Galanos, and Jiri Bartek. 2023 “MDC1 Maintains Active Elongation Complexes of RNA Polymerase II.” Cell Reports 42(1):111979. doi: 10.1016/j.celrep.2022.111979.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL 355/14 (UGA-42 Caliburn). - van den Heuvel, Diana, Mihyun Kim, Annelotte P. Wondergem, Paula J. van der Meer, Myrèse Witkamp, Ferdy Lambregtse, Hyun-Suk Kim, Folkert Kan, Katja Apelt, Angela Kragten, Román González-Prieto, Alfred C. O. Vertegaal, Jung-Eun Yeo, Byung-Gyu Kim, Remco van Doorn, Orlando D. Schärer, and Martijn S. Luijsterburg. 2023 “A Disease-Associated XPA Allele Interferes with TFIIH Binding and Primarily Affects Transcription-Coupled Nucleotide Excision Repair.” Proceedings of the National Academy of Sciences 120(11):2017. doi: 10.1073/pnas.2208860120.
Equipment used: Photomanipulation system UGA-42 Caliburn 2L with 2 pulsed UV laser DPSL-266 and DPSL-355 (UGA-42 Caliburn).
2022
- Blessing, Charlotte, Katja Apelt, Diana van den Heuvel, Claudia Gonzalez-Leal, Magdalena B. Rother, Melanie van der Woude, Román González-Prieto, Adi Yifrach, Avital Parnas, Rashmi G. Shah, Tia Tyrsett Kuo, Daphne E. C. Boer, Jin Cai, Angela Kragten, Hyun-suk Kim, Orlando D. Schärer, Alfred C. O. Vertegaal, Girish M. Shah, Sheera Adar, Hannes Lans, Haico van Attikum, Andreas G. Ladurner, and Martijn S. Luijsterburg. 2022 “XPC–PARP Complexes Engage the Chromatin Remodeler ALC1 to Catalyze Global Genome DNA Damage Repair.” Nature Communications 13(1):4762. doi: 10.1038/s41467-022-31820-4.
Equipment used: Photomanipulation system UGA-42 Caliburn 2L with 2 pulsed UV laser DPSL-266 and DPSL-355 (UGA-42 Caliburn). - Chansard, Audrey, Enrico Pobega, Pierre Caron, and Sophie E. Polo. 2022 “Imaging the Response to DNA Damage in Heterochromatin Domains.” Frontiers in Cell and Developmental Biology 10(June):1–10. doi: 10.3389/fcell.2022.920267.
Equipment used: Spot illumination module with pulsed UV laser DPSS-266 (AiWon Caliburn) - Corbeski, Ivan, Xiaohu Guo, Bruna V Eckhardt, Domenico Fasci, Wouter Wiegant, Melissa A. Graewert, Kees Vreeken, Hans Wienk, Dmitri I. Svergun, Albert J. R. Heck, Haico van Attikum, Rolf Boelens, Titia K. Sixma, Francesca Mattiroli, and Hugo van Ingen. “Chaperoning of the Histone Octamer by the Acidic Domain of DNA Repair Factor APLF.” Science Advances 8(30):1–16. doi: 10.1126/sciadv.abo0517.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355/14 (UGA-42 Caliburn) - Gómez-Cabello, Daniel, George Pappas, Diana Aguilar-Morante, Christoffel Dinant, and Jiri Bartek. 2022 “CtIP-Dependent Nascent RNA Expression Flanking DNA Breaks Guides the Choice of DNA Repair Pathway.” Nature Communications 13(1):1–15. doi: 10.1038/s41467-022-33027-z.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355/14 (UGA-42 Caliburn). - Lee, Doohyung, Katja Apelt, Seong-ok Lee, Hsin-ru Chan, Martijn S. Luijsterburg, Justin W. C. Leung, and Kyle M. Miller. 2022 “ZMYM2 Restricts 53BP1 at DNA Double-Strand Breaks to Favor BRCA1 Loading and Homologous Recombination.” Nucleic Acids Research 1–22. doi: 10.1093/nar/gkac160.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355/14 (UGA-42 Caliburn). - Schubert, Lisa, Ivo A. Hendriks, Emil P. T. Hertz, Wei Wu, Selene Sellés‐Baiget, Saskia Hoffmann, Keerthana Stine Viswalingam, Irene Gallina, Satyakrishna Pentakota, Bente Benedict, Joachim Johansen, Katja Apelt, Martijn S. Luijsterburg, Simon Rasmussen, Michael Lisby, Ying Liu, Michael L. Nielsen, Niels Mailand, and Julien P. Duxin. 2022 “SCAI Promotes Error‐free Repair of DNA Interstrand Crosslinks via the Fanconi Anemia Pathway.” EMBO Reports 1–20. doi: 10.15252/embr.202153639.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266/5 (UGA-42 Caliburn) - Tsaridou, Stavroula, Georgia Velimezi, Frances Willenbrock, Maria Chatzifrangkeskou, Waheba Elsayed, Andreas Panagopoulos, Dimitris Karamitros, Vassilis Gorgoulis, Zoi Lygerou, Vassilis Roukos, Eric O’Neill, and Dafni Eleftheria Pefani. “53BP1-Mediated Recruitment of RASSF1Ato Ribosomal DNA Breaks Promotes LocalATM Signaling.” EMBO Reports 1–21. doi: 10.15252/embr.202154483.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 355nm UV laser (UGA-42 Caliburn) - van Toorn, Marvin, Yasemin Turkyilmaz, Sueji Han, Di Zhou, Hyun-Suk Kim, Irene Salas-Armenteros, Mihyun Kim, Masaki Akita, Franziska Wienholz, Anja Raams, Eunjin Ryu, Sukhyun Kang, Arjan F. Theil, Karel Bezstarosti, Maria Tresini, Giuseppina Giglia-Mari, Jeroen A. Demmers, Orlando D. Schärer, Jun-Hyuk Choi, Wim Vermeulen, and Jurgen A. Marteijn. 2022 “Active DNA Damage Eviction by HLTF Stimulates Nucleotide Excision Repair.” Molecular Cell. doi: 10.1016/j.molcel.2022.02.020.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355 (UGA-42 Caliburn). - Wang, Yu-Hsiu, Teresa L. F. Ho, Anushya Hariharan, Hui Chin Goh, Yao Liang Wong, Nicole S. Verkaik, May Yin Lee, Wai Leong Tam, Dik C. van Gent, Ashok R. Venkitaraman, Michael P. Sheetz, and David P. Lane. 2022 “Rapid Recruitment of P53 to DNA Damage Sites Directs DNA Repair Choice and Integrity.” Proceedings of the National Academy of Sciences 119(10):1–13. doi: 10.1073/pnas.2113233119.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355 (UGA-42 Caliburn).
2021
- Apelt, Katja, Susan M. White, Hyun Suk Kim, Jung Eun Yeo, Angela Kragten, Annelotte P. Wondergem, Martin A. Rooimans, Román González-Prieto, Wouter W. Wiegant, Sebastian Lunke, Daniel Flanagan, Sarah Pantaleo, Catherine Quinlan, Winita Hardikar, Haico van Attikum, Alfred C. O. Vertegaal, Brian T. Wilson, Rob M. F. Wolthuis, Orlando D. Schärer, and Martijn S. Luijsterburg. 2021 “ERCC1 Mutations Impede DNA Damage Repair and Cause Liver and Kidney Dysfunction in Patients.” The Journal of Experimental Medicine 218(3).
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266 and DPSL-355/14 (UGA-42 Caliburn) - Khanam, Taran, Ivan Muñoz, Florian Weiland, Thomas Carroll, Michael Morgan, Barbara N. Borsos, Vasiliki Pantazi, Meghan Slean, Miroslav Novak, Rachel Toth, Paul Appleton, Tibor Pankotai, Houjiang Zhou, and John Rouse. 2021 “CDKL5 Kinase Controls Transcription‐coupled Responses to DNA Damage.” The EMBO Journal 1–25. doi: 10.15252/embj.2021108271.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Madhavan, Bindhu K., Zhe Han, Albert Sickmann, Rainer Pepperkok, Peter P. Nawroth, and Varun Kumar. 2021 “A Laser-Mediated Photo-Manipulative Toolbox for Generation and Real-Time Monitoring of DNA Lesions.” STAR Protocols 2(3):100700. doi: 10.1016/j.xpro.2021.100700.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355/14 (UGA-42 Caliburn) and several others mentioned: “Laser setup available (DPSL-266, DPSS-355/14, DPSL-355, DL-405)” - Pfeiffer, Annika, Laura K. Herzog, Martijn S. Luijsterburg, Rashmi G. Shah, Magdalena B. Rother, Henriette Stoy, Ulrike Kühbacher, Haico van Attikum, Girish M. Shah, and Nico P. Dantuma. 2021. “Poly(ADP-Ribosyl)Ation Temporally Confines SUMO-Dependent Ataxin-3 Recruitment to Control DNA Double-Strand Break Repair.” Journal of Cell Science jcs.247809.
Equipment used: Spot illumination module with pulsed UV laser DPSS-355 (AiWon Caliburn) - Sabatella, Mariangela, Karen L. Thijssen, Carlota Davó-Martínez, Wim Vermeulen, and Hannes Lans. 2021. “Tissue-Specific DNA Repair Activity of ERCC-1/XPF-1.” Cell Reports 34(2):108608.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266 (UGA-42 Caliburn)
2020
- Apelt, Katja, Iris Zoutendijk, Dennis Y. Gout, Annelotte P. Wondergem, Diana van den Heuvel, and Martijn S. Luijsterburg. 2020. “Human HMGN1 and HMGN2 Are Not Required for Transcription-Coupled DNA Repair.” Scientific Reports 10(1):4332.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266/5 (UGA-42 Caliburn) - Blessing, Charlotte, Imke Karlijn Mandemaker, Claudia Gonzalez-Leal, Julia Preisser, Adrian Schomburg, and Andreas Gerhard Ladurner. “The Oncogenic Helicase ALC1 Regulates PARP Inhibitor Potency by Trapping PARP2 at DNA Breaks.” Molecular Cell 80(5):862-875.e6.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355 (UGA-42 Caliburn). - Liebelt, Frauke, Joost Schimmel, Matty Verlaan-de Vries, Esra Klemann, Martin E. van Royen, Yana van der Weegen, Martijn S. Luijsterburg, Leon H. Mullenders, Alex Pines, Wim Vermeulen, and Alfred C. O. Vertegaal. 2020. “Transcription-Coupled Nucleotide Excision Repair Is Coordinated by Ubiquitin and SUMO in Response to Ultraviolet Irradiation.” Nucleic Acids Research 48(1):231–48.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266/2 (UGA-42 Caliburn) - Ribeiro-Silva, Cristina, Mariangela Sabatella, Angela Helfricht, Jurgen A. Marteijn, Arjan F. Theil, Wim Vermeulen, and Hannes Lans. 2020. “Ubiquitin and TFIIH-Stimulated DDB2 Dissociation Drives DNA Damage Handover in Nucleotide Excision Repair.” Nature Communications 11(1):1–14.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 2 mW UV laser DPSL-266/2 and 4.5 mW UV laser DPSL 266/5 (UGA-42 Caliburn)
2019
- Boonen, Rick A. C. M., Amélie Rodrigue, Chantal Stoepker, Wouter W. Wiegant, Bas Vroling, Milan Sharma, Magdalena B. Rother, Nandi Celosse, Maaike P. G. Vreeswijk, Fergus Couch, Jacques Simard, Peter Devilee, Jean Yves Masson, and Haico van Attikum. 2019. “Functional Analysis of Genetic Variants in the High-Risk Breast Cancer Susceptibility Gene PALB2.” Nature Communications 10(1):1–15.
Equipment used: Photomanipulation system AiWon with pulsed UV laser DPSS-355 (AiWon Caliburn) - Pacquelet, Anne, Matthieu Jousseaume, Jocelyn Etienne, and Grégoire Michaux. 2019. “Simultaneous Regulation of Cytokinetic Furrow and Nucleus Positions by Cortical Tension Contributes to Proper DNA Segregation during Late Mitosis.” Current Biology 29(22):3766-3777.e4.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-355 (UGA-42 Caliburn) - Wienholz, Franziska, Di Zhou, Yasemin Turkyilmaz, Petra Schwertman, Maria Tresini, Alex Pines, Marvin Van Toorn, Karel Bezstarosti, Jeroen A. A. Demmers, and Jurgen A. Marteijn. 2019. “FACT Subunit Spt16 Controls UVSSA Recruitment to Lesion-Stalled RNA Pol II and Stimulates TC-NER.” Nucleic Acids Research 47(8):4011–25.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266/2 (UGA-42 Caliburn)
2018
- Ribeiro-Silva, Cristina, Özge Z. Aydin, Raquel Mesquita-Ribeiro, Jana Slyskova, Angela Helfricht, Jurgen A. Marteijn, Jan H. J. Hoeijmakers, Hannes Lans, and Wim Vermeulen. 2018. “DNA Damage Sensitivity of SWI/SNF-Deficient Cells Depends on TFIIH Subunit P62/GTF2H1.” Nature Communications 9(1).
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266 (UGA-42 Caliburn) - Smith, Rebecca and Gyula Timinszky. 2018. “Monitoring Poly(ADP-Ribosyl)Ation in Response to DNA Damage in Live Cells Using Fluorescently Tagged Macrodomains.” Pp. 11–24 in.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Smith, Rebecca, Hafida Sellou, Catherine Chapuis, Sébastien Huet, and Gyula Timinszky. 2018. “CHD3 and CHD4 Recruitment and Chromatin Remodeling Activity at DNA Breaks Is Promoted by Early Poly(ADP-Ribose)-Dependent Chromatin Relaxation.” Nucleic Acids Research 46(12):6087–98.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Schmalz, Michael F., Ines Wieser, Felix Schindler, Christina Czada, Alfred Leitenstorfer, and Elisa Ferrando-May. “Highly Standardized Multicolor Femtosecond Fiber System for Selective Microphotomanipulation of Deoxyribonucleic Acid and Chromatin.” Optics Letters 43(12):2877. doi: 10.1364/ol.43.002877.
Equipment used: Photomanipulation system UGA-42 Firefly with femtosecond-pulsed laser (UGA-42 Caliburn)
2017
- Golia, Barbara, Giuliana Katharina Moeller, Gytis Jankevicius, Andreas Schmidt, Anna Hegele, Julia Preißer, Mai Ly Tran, Axel Imhof, and Gyula Timinszky. 2017. “ATM Induces MacroD2 Nuclear Export upon DNA Damage.” Nucleic Acids Research 45(1):244–54.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Lebeaupin, Théo, Rebecca Smith, Sébastien Huet, and Gyula Timinszky. 2017. “Poly(ADP-Ribose)-Dependent Chromatin Remodeling in DNA Repair.” Pp. 165–83 in.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Singh, Hari R., Aurelio P. Nardozza, Ingvar R. Möller, Gunnar Knobloch, Hans A. V. Kistemaker, Markus Hassler, Nadine Harrer, Charlotte Blessing, Sebastian Eustermann, Christiane Kotthoff, Sébastien Huet, Felix Mueller-Planitz, Dmitri V. Filippov, Gyula Timinszky, Kasper D. Rand, and Andreas G. Ladurner. 2017. “A Poly-ADP-Ribose Trigger Releases the Auto-Inhibition of a Chromatin Remodeling Oncogene.” Molecular Cell 68(5):860–871.e7.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn)
2016
- Rank, Lisa, Sebastian Veith, Eva C. Gwosch, Janine Demgenski, Magdalena Ganz, Marjolijn C. Jongmans, Christopher Vogel, Arthur Fischbach, Stefanie Buerger, Jan M. F. Fischer, Tabea Zubel, Anna Stier, Christina Renner, Michael Schmalz, Sascha Beneke, Marcus Groettrup, Roland P. Kuiper, Alexander Bürkle, Elisa Ferrando-May, and Aswin Mangerich. 2016. “Analyzing Structure-Function Relationships of Artificial and Cancer-Associated PARP1 Variants by Reconstituting TALEN-Generated HeLa PARP1 Knock-out Cells.” Nucleic Acids Research 44(21):10386–405.
Equipment used: Photomanipulation system UGA-42 Firefly with femtosecond-pulsed fiber laser 780 nm (UGA-42 Caliburn) - Sellou, H., T. Lebeaupin, C. Chapuis, R. Smith, A. Hegele, H. R. Singh, M. Kozlowski, S. Bultmann, A. G. Ladurner, G. Timinszky, and S. Huet. 2016. “The Poly(ADP-Ribose)-Dependent Chromatin Remodeler Alc1 Induces Local Chromatin Relaxation upon DNA Damage.” Molecular Biology of the Cell 27(24):3791–99.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405
2015
- Aydin, Özge Z., Jurgen A. Marteijn, Cristina Ribeiro-Silva, Aida Rodríguez López, Nils Wijgers, Godelieve Smeenk, Haico van Attikum, Raymond A. Poot, Wim Vermeulen, and Hannes Lans. 2014. “Human ISWI Complexes Are Targeted by SMARCA5 ATPase and SLIDE Domains to Help Resolve Lesion-Stalled Transcription.” Nucleic Acids Research 42 (13): 8473–85.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed UV laser DPSL-266 (UGA-42 Caliburn) - Tresini, Maria, Daniël O. Warmerdam, Petros Kolovos, Loes Snijder, Mischa G. Vrouwe, Jeroen A. A. Demmers, Wilfred F. J. Van Ijcken, Frank G. Grosveld, René H. Medema, Jan H. J. Hoeijmakers, Leon H. F. Mullenders, Wim Vermeulen, and Jurgen A. Marteijn.2015. “The Core Spliceosome as Target and Effector of Non-Canonical ATM Signalling.” Nature 523(7558):53–58.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 2mW UV laser DPSL-266/2 (UGA-42 Caliburn) - Van Cuijk, Loes, Gijsbert J. Van Belle, Yasemin Turkyilmaz, Sara L. Poulsen, Roel C. Janssens, Arjan F. Theil, Mariangela Sabatella, Hannes Lans, Niels Mailand, Adriaan B. Houtsmuller, Wim Vermeulen, and Jurgen A. Marteijn. 2015. “SUMO and Ubiquitin-Dependent XPC Exchange Drives Nucleotide Excision Repair.” Nature Communications 6(May).
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 2 mW UV laser DPSL-266/2 (UGA-42 Caliburn)
2013
- Dinant, Christoffel, Giannis Ampatziadis-Michailidis, Hannes Lans, Maria Tresini, Anna Lagarou, Malgorzata Grosbart, ArjanF Theil, WiggertA VanCappellen, Hiroshi Kimura, Jiri Bartek, Maria Fousteri, AdriaanB Houtsmuller, Wim Vermeulen, and JurgenA Marteijn. 2013. “Enhanced Chromatin Dynamics by Fact Promotes Transcriptional Restart after UV-Induced DNA Damage.” Molecular Cell 51(4):469–79.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn) - Jankevicius, Gytis, Markus Hassler, Barbara Golia, Vladimir Rybin, Martin Zacharias, Gyula Timinszky, and Andreas G. Ladurner. 2013. “A Family of Macrodomain Proteins Reverses Cellular Mono-ADP-Ribosylation.” Nature Structural and Molecular Biology 20(4):508–14.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Poulsen, Sara L., Rebecca K. Hansen, Sebastian A. Wagner, Loes van Cuijk, Gijsbert J. van Belle, Werner Streicher, Mats Wikström, Chunaram Choudhary, Adriaan B. Houtsmuller, Jurgen A. Marteijn, Simon Bekker-Jensen, Niels Mailand . 2013. “RNF111/Arkadia Is a SUMO-Targeted Ubiquitin Ligase That Facilitates the DNA Damage Response.” Journal of Cell Biology 201 (6): 797–807.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn) - Theil, Arjan F., Julie Nonnekens, Barbara Steurer, Pierre-Olivier Mari, Jan de Wit, Charlène Lemaitre, Jurgen a Marteijn, Anja Raams, Alex Maas, Marcel Vermeij, Jeroen Essers, Jan H. J. Hoeijmakers, Giuseppina Giglia-Mari, and Wim Vermeulen. 2013. “Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality.” PLoS Genetics 9(4):e1003431.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn)
2012
- Bergink, Steven, Wendy Toussaint, Martijn S. Luijsterburg, Christoffel Dinant, Sergey Alekseev, Jan H. J. Hoeijmakers, Nico P. Dantuma, Adriaan B. Houtsmuller, and Wim Vermeulen. 2012. “Recognition of DNA Damage by XPC Coincides with Disruption of the XPC-RAD23 Complex.” Journal of Cell Biology 196(6):681–88.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn) - Schwertman, Petra, Anna Lagarou, Dick H. W. Dekkers, Anja Raams, Adriana C. van der Hoek, Charlie Laffeber, Jan H. J. Hoeijmakers, Jeroen a a Demmers, Maria Fousteri, Wim Vermeulen, and Jurgen a Marteijn. 2012. “UV-Sensitive Syndrome Protein UVSSA Recruits USP7 to Regulate Transcription-Coupled Repair.” Nature Genetics 44(5):598–602.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn)
2011
- Vermeulen, Wim. 2011. “Dynamics of Mammalian NER Proteins.” DNA Repair 10(7):760–71.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn)
2009
- Luijsterburg, Martijn S., Christoffel Dinant, Hannes Lans, Jan Stap, Elzbieta Wiernasz, Saskia Lagerwerf, Daniël O. Warmerdam, Michael Lindh, Maartje C. Brink, Jurek W. Dobrucki, Jacob a Aten, Maria I. Fousteri, Gert Jansen, Nico P. Dantuma, Wim Vermeulen, Leon H. F. Mullenders, Adriaan B. Houtsmuller, Pernette J. Verschure, and Roel van Driel. 2009. “Heterochromatin Protein 1 Is Recruited to Various Types of DNA Damage.” The Journal of Cell Biology 185(4):577–86.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn)
2007
- Dinant, Christoffel, Martijn de Jager, Jeroen Essers, Wiggert a van Cappellen, Roland Kanaar, Adriaan B. Houtsmuller, and Wim Vermeulen. 2007. “Activation of Multiple DNA Repair Pathways by Sub-Nuclear Damage Induction Methods.” Journal of Cell Science 120(Pt 15):2731–40.
Equipment used: Spot illumination module with pulsed 2mW UV laser DPSL-266/2 (AiWon Caliburn)
IR & IR Temperature Jump
IR
2023
- Ramey-Ward, Allison N., Yixiao Dong, Jin Yang, Hiroaki Ogasawara, Elizabeth C. Bremer-Sai, Olga Brazhkina, Christian Franck, Michael Davis, and Khalid Salaita. 2023. “Optomechanically Actuated Hydrogel Platform for Cell Stimulation with Spatial and Temporal Resolution.” ACS Biomaterials Science and Engineering. doi: 10.1021/acsbiomaterials.3c00516.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-830
2021
- Zhu, Dingcheng, Lili Feng, Neus Feliu, Andreas H. Guse, and Wolfgang J. Parak. 2021. “Stimulation of Local Cytosolic Calcium Release by Photothermal Heating for Studying Intra- and Intercellular Calcium Waves.” Advanced Materials 2008261. doi: 10.1002/adma.202008261.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-830
2020
- Ramey-Ward, Allison N., Hanquan Su, and Khalid Salaita. 2020. “Mechanical Stimulation of Adhesion Receptors Using Light-Responsive Nanoparticle Actuators Enhances Myogenesis.” ACS Applied Materials & Interfaces 12(32):35903–17.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-785
2018
- Su, Hanquan, Zheng Liu, Yang Liu, Victor Pui-Yan Ma, Aaron Blanchard, Jing Zhao, Kornelia Galior, R. Brian Dyer, and Khalid Salaita. 2018. “Light-Responsive Polymer Particles as Force Clamps for the Mechanical Unfolding of Target Molecules.” Nano Letters 18(4):2630–36.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-785 - Kantner, Karsten, Joanna Rejman, Karl V. L. Kraft, Mahmoud G. Soliman, Mikhail V. Zyuzin, Alberto Escudero, Pablo del Pino, and Wolfgang J. Parak. 2018. “Laterally and Temporally Controlled Intracellular Staining by Light-Triggered Release of Encapsulated Fluorescent Markers.” Chemistry – A European Journal 24(9):2098–2102. doi: 10.1002/chem.201706135.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-830
FLIRT
2020
- Rosenberger, Daniela C., Uta Binzen, Rolf Detlef Treede, and Wolfgang Greffrath. 2020. “The Capsaicin Receptor TRPV1 Is the First Line Defense Protecting from Acute Non Damaging Heat: A Translational Approach.” Journal of Translational Medicine 18(1):1–16.
Equipment used:Photomanipulation system UGA-42 with diode single line laser DL-1470
2018
- Hirsch, Sophia M., Sriramkumar Sundaramoorthy, Tim Davies, Yelena Zhuravlev, Jennifer C. Waters, Mimi Shirasu-Hiza, Julien Dumont, and Julie C. Canman. 2018. “FLIRT: Fast Local Infrared Thermogenetics for Subcellular Control of Protein Function.” Nature Methods.
Equipment used: Photomanipulation system UGA-42 Geo with diode single line laser DL-1470
IR-LEGO
2023
- Zilliox, Marie, Vanessa Tillement, Thomas Mangeat, Sophie Polès, Patrick Blader, and Julie Batut. 2023. “Protocol to Locally Express Cxcl12a during Zebrafish Olfactory Organ Development by Combining IR-LEGO with Live Imaging.” STAR Protocols 4(3):102538. doi: 10.1016/j.xpro.2023.102538.
Equipment used:Photomanipulation system UGA-42 with diode single line laser DL-1470
2017
- Sundaramoorthy, Sriramkumar, Adrian Garcia Badaracco, Sophia M. Hirsch, Jun Hong Park, Tim Davies, Julien Dumont, Mimi Shirasu-Hiza, Andrew C. Kummel, and Julie C. Canman. 2017. “Low Efficiency Upconversion Nanoparticles for High-Resolution Coalignment of Near-Infrared and Visible Light Paths on a Light Microscope.” ACS Applied Materials & Interfaces 9(9):7929–40.
Equipment used: Photomanipulation system UGA-42 Geo with diode single line laser DL-1470
2009
- Suzuki, Motoshi, Yasuhiro Kamei, Shunsuke Yuba, and Shin Takagi. 2009. “Manipulating Behaviors of Targeted Single Cells in Vivo by Using IR-LEGO.” 20th Anniversary MHS 2009 and Micro-Nano Global COE – 2009 International Symposium on Micro-NanoMechatronics and Human Science 326–31.
Equipment used: Photomanipulation system UGA-42 Geo with diode single line laser DL-1470
Uncaging & Photolysis
2023
- Csemer, Andrea, Adrienn Kovács, Baneen Maamrah, Krisztina Pocsai, Kristóf Korpás, Álmos Klekner, Péter Szücs, Péter P. Nánási, and Balázs Pál. 2023 “Astrocyte- and NMDA Receptor-Dependent Slow Inward Currents Differently Contribute to Synaptic Plasticity in an Age-Dependent Manner in Mouse and Human Neocortex.” Aging Cell (December 2022):1–19. doi: 10.1111/acel.13939.
Equipment used: Flash lamp system JML-C2 - Grabner, Chad P., Daiki Futagi, Jun Shi, Vytas Bindokas, Katsunori Kitano, Eric A. Schwartz, and Steven H. DeVries. 2023. “Mechanisms of Simultaneous Linear and Nonlinear Computations at the Mammalian Cone Photoreceptor Synapse.” Nature Communications 14(1). doi: 10.1038/s41467-023-38943-2.
Equipment used: Spot illumination module (AiWon). - Vélez-Ortega, A. Catalina, Ruben Stepanyan, Stephanie E. Edelmann, Sara Torres-Gallego, Channy Park, Desislava A. Marinkova, Joshua S. Nowacki, Ghanshyam P. Sinha, and Gregory I. Frolenkov. 2023 “TRPA1 Activation in Non-Sensory Supporting Cells Contributes to Regulation of Cochlear Sensitivity after Acoustic Trauma.” Nature Communications 14(1):3871. doi: 10.1038/s41467-023-39589-w.
Equipment used: DPSL 355/30 laser. - Weichselbaum, Ewald, Timur Galimzyanov, Oleg V Batishchev, Sergey A. Akimov, and Peter Pohl. 2023 “Proton Migration on Top of Charged Membranes.” Biomolecules 13(2):352. doi: 10.3390/biom13020352.
Equipment used: Flash lamp system JML-C2
2022
- Comini, Maddalena, Juan Sierra-Marquez, Gustavo A. Guzman, Arne Franzen, Antje Willuweit, Istvan Katona, Patricia Hidalgo, Christoph Fahlke, and Raul E. Guzman. 2022 “CLC Anion/Proton Exchangers Regulate Secretory Vesicle Filling and Granule Exocytosis in Chromaffin Cells.” The Journal of Neuroscience JN-RM-2439-21. doi: 10.1523/JNEUROSCI.2439-21.2022.
Equipment used: Flash lamp system JML-C2 - Gruteser, Nadine, and Arnd Baumann. 2022 “Examination of Intracellular GPCR-Mediated Signaling with High Temporal Resolution.” International Journal of Molecular Sciences 23(15):8516. doi: 10.3390/ijms23158516.
Equipment used: Flash lamp system JML-C2 - Gutorov, Rita, Ben Katz, and Baruch Minke. “Electrophysiological Methods for Measuring Photopigment Levels in Drosophila Photoreceptors.” Journal of Visualized Experiments (184). doi: 10.3791/63514.
Equipment used: Flash lamp system JML-C2 - Maul, Alena, Antje Kathrin Huebner, Nicola Strenzke, Tobias Moser, Rudolf Rübsamen, Saša Jovanovic, and Christian A. Hübner. 2022 “The Cl–Channel TMEM16A Is Involved in the Generation of Cochlear Ca2+ Waves and Promotes the Refinement of Auditory Brainstem Networks in Mice.” ELife 11:1–23. doi: 10.7554/elife.72251.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with 405nm laser - Ravasenga, Tiziana, Massimo Ruben, Vincenzo Regio, Alice Polenghi, Enrica Maria Petrini, and Andrea Barberis. 2022 “Spatial Regulation of Coordinated Excitatory and Inhibitory Synaptic Plasticity at Dendritic Synapses.” Cell Reports 38(6):110347. doi: 10.1016/j.celrep.2022.110347.
Equipment used: Photomanipulation system (UGA-42 Firefly) - Staudt, Angelina, Olga Ratai, Aicha Bouzouina, Claudia Fecher-Trost, Ahmed Shaaban, Hawraa Bzeih, Alexander Horn, Ali H. Shaib, Margarete Klose, Veit Flockerzi, Marcel A. Lauterbach, Jens Rettig, and Ute Becherer. 2022 “Localization of the Priming Factors CAPS1 and CAPS2 in Mouse Sensory Neurons Is Determined by Their N-Termini.” Frontiers in Molecular Neuroscience 15(April):1–21. doi: 10.3389/fnmol.2022.674243.
Equipment used: Flash lamp system JML-C2
2021
- Capel, Rebecca A., Samuel J. Bose, Thomas P. Collins, Skanda Rajasundaram, Thamali Ayagama, Manuela Zaccolo, Rebecca Ann Beatrice Burton, and Derek A. Terrar. 2021 “IP3-Mediated Ca2 þ Release Regulates Atrial Ca2 þ Transients and Pacemaker Function by Stimulation of Adenylyl Cyclases.” American Journal of Physiology – Heart and Circulatory Physiology 320(1):H95–107. doi: 10.1152/AJPHEART.00380.2020.
Equipment used: Flash lamp system JML-C2 - Eshra, Abdelmoneim, Hartmut Schmidt, Jens Eilers, and Stefan Hallermann. 2021 “Calcium Dependence of Neurotransmitter Release at a High Fidelity Synapse.” ELife 10:1–34. doi: 10.7554/eLife.70408.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-375/200 - Ghezzi, Filippo, Andre Marques-Smith, Paul G. Anastasiades, Daniel Lyngholm, Cristiana Vagnoni, Alexandra Rowett, Gokul Parameswaran, Anna Hoerder-Suabedissen, Yasushi Nakagawa, Zoltan Molnar, and Simon JB Butt. 2021. “Non-Canonical Role for Lpar1-EGFP Subplate Neurons in Early Postnatal Mouse Somatosensory Cortex.” ELife 10:2020.05.12.088450. doi: 10.7554/eLife.60810.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with 355 nm pulsed DPSL laser 355/30 - Houy, Sébastien, Joana S. Martins, Noa Lipstein, and Jakob Balslev Sørensen. 2022. “Phorbolester-Activated Munc13-1 and UbMunc13-2 Exert Opposing Effects on Dense-Core Vesicle Secretion.” ELife 11:1–26. doi: 10.7554/ELIFE.79433.
Equipment used: Flash lamp system JML-C2 - Nuno-Perez, Alvaro, Massimo Trusel, Arnaud L. Lalive, Mauro Congiu, Denise Gastaldo, Anna Tchenio, Salvatore Lecca, Mariano Soiza-Reilly, Claudia Bagni, and Manuel Mameli. 2021 “Stress Undermines Reward-Guided Cognitive Performance through Synaptic Depression in the Lateral Habenula.” Neuron 1–10. doi: 10.1016/j.neuron.2021.01.008.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-405 - Pampaloni, Niccolò P., Irene Riva, Anna L. Carbone, and Andrew J. R. Plested. 2021. “Slow AMPA Receptors in Hippocampal Principal Cells.” Cell Reports 36(5):109496. doi: 10.1016/j.celrep.2021.109496.
Equipment used: Photomanipulation system (UGA-42 Firefly) with diode single line laser DL-405 - Plested, Andrew J. R., and Mette H. Poulsen. “Crosslinking Glutamate Receptor Ion Channels.” Pp. 161–92 in Methods in Enzymology. Elsevier Inc.
Equipment described Flash lamp system JML-C2; high power short wave UV illumination system UVICO (UVICO-2) & Illumination system KSL-2 with UV light 365nm (KSL-2) - Tawfik, Bassam, Joana S. Martins, Sébastien Houy, Cordelia Imig, Paulo S. Pinheiro, Sonja M. Wojcik, Nils Brose, Benjamin H. Cooper, and Jakob Balslev Sørensen. 2021. “Synaptotagmin-7 Places Dense-Core Vesicles at the Cell Membrane to Promote Munc13-2-and Ca2+-Dependent Priming.” ELife 10: 1–40. https://doi.org/10.7554/eLife.64527.
Equipment used: Flash lamp system JML-C2
2020
- Buckley, Charlotte, Calum Wilson, and John G. McCarron. 2020 “FK506 Regulates Ca2+ Release Evoked by Inositol 1,4,5-Trisphosphate Independently of FK-Binding Protein in Endothelial Cells.” British Journal of Pharmacology 177(5):1131–49.
Equipment used: Flash lamp system JML-C2 - Buckley, Charlotte, Xun Zhang, Calum Wilson, and John G. McCarron. 2020. “Carbenoxolone and 18β‐Glycyrrhetinic Acid Inhibit IP 3 ‐Mediated Endothelial Cell Calcium Signalling and Depolarise Mitochondria.” British Journal of Pharmacology bph.15329.
Equipment used: Flash lamp system JML-C2 - Gomez, Laura C., Shin-ya Kawaguchi, Thibault Collin, Abdelali Jalil, Maria del Pilar Gomez, Enrico Nasi, Alain Marty, and Isabel Llano. 2020. “Influence of Spatially Segregated IP 3 -Producing Pathways on Spike Generation and Transmitter Release in Purkinje Cell Axons.” Proceedings of the National Academy of Sciences 117(20):11097–108. doi: 10.1073/pnas.2000148117.
Equipment used: Photomanipulation system (UGA-42 Firefly) with diode single line laser DL-405 - Gowrisankaran, Sindhuja, Sébastien Houy, Johanna G. Peñ. del Castillo, Vicky Steubler, Monika Gelker, Jana Kroll, Paulo S. Pinheiro, Dirk Schwitters, Nils Halbsgut, Arndt Pechstein, Jan R. T. van Weering, Tanja Maritzen, Volker Haucke, Nuno Raimundo, Jakob B. Sørensen, and Ira Milosevic. 2020. “Endophilin-A Coordinates Priming and Fusion of Neurosecretory Vesicles via Intersectin.” Nature Communications 11(1).
Equipment used: Flash lamp system JML-C2 - Liang, Tao, Tairan Qin, Fei Kang, Youhou Kang, Li Xie, Dan Zhu, Subhankar Dolai, Dafna Greitzer-Antes, Robert K. Baker, Daorong Feng, Eva Tuduri, Claes Goran Ostenson, Timothy J. Kieffer, Kate Banks, Jeffrey E. Pessin, and Herbert Y. Gaisano. 2020. “SNAP23 Depletion Enables More SNAP25/Calcium Channel Excitosome Formation to Increase Insulin Exocytosis in Type 2 Diabetes.” JCI Insight 5(3).
Equipment used: Flash lamp system JML-C2 - Polenghi, Alice, Thierry Nieus, Stefania Guazzi, Pau Gorostiza, Enrica Maria Petrini, and Andrea Barberis. 2020. “Kainate Receptor Activation Shapes Short-Term Synaptic Plasticity by Controlling Receptor Lateral Mobility at Glutamatergic Synapses.” Cell Reports 31(10):107735.
Equipment used: Photomanipulation system (UGA-42 Firefly). - Ramírez-Gómez, Héctor Vicente, Vilma Jimenez Sabinina, Martín Velázquez Pérez, Carmen Beltran, Jorge Carneiro, Christopher D. Wood, Idan Tuval, Alberto Darszon, and Adán Guerrero. 2020. “Sperm Chemotaxis Is Driven by the Slope of the Chemoattractant Concentration Field.” ELife 9:1–32.
Equipment used: high power short wave UV illumination system UVICO (UVICO-2) - Xian, Wenying, Hongmei Wang, Alessandra Moretti, Karl Ludwig Laugwitz, Veit Flockerzi, and Peter Lipp. 2020. “Domain Zipping and Unzipping Modulates TRPM4’s Properties in Human Cardiac Conduction Disease.” FASEB Journal (June):12114–26.
Equipment used: Flash lamp system JML-C2
2019
- Denwood, Geoffrey, Andrei Tarasov, Albert Salehi, Elisa Vergari, Reshma Ramracheya, Harumi Takahashi, Viacheslav O. Nikolaev, Susumo Seino, Fiona Gribble, Frank Reimann, Patrik Rorsman, and Quan Zhang. 2019. “Glucose Stimulates Somatostatin Secretion in Pancreatic δ-Cells by CAMP-Dependent Intracellular Ca2+ Release.” The Journal of General Physiology 151(9):1094–1115.
Equipment used: Flash lamp system JML-C2 - Grushevskyi, Eugene O., Taulant Kukaj, Ralf Schmauder, Andreas Bock, Ulrike Zabel, Tina Schwabe, Klaus Benndorf, and Martin J. Lohse. 2019. “Stepwise Activation of a Class C GPCR Begins with Millisecond Dimer Rearrangement.” Proceedings of the National Academy of Sciences of the United States of America 116(20):10150–55.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-375 - Heathcote, Helen R., Matthew D. Lee, Xun Zhang, Christopher D. Saunter, Calum Wilson, and John G. McCarron. “Endothelial TRPV4 Channels Modulate Vascular Tone by Ca2+-Induced Ca2+ Release at Inositol 1,4,5-Trisphosphate Receptors.” British Journal of Pharmacology 176(17):3297–3317. doi: 10.1111/bph.14762.
Equipment used: Flash lamp system JML-C2 - Kong, Deqing, Zhiyi Lv, Matthias Häring, Benjamin Lin, Fred Wolf, and Jörg Großhans. 2019. “In Vivo Optochemical Control of Cell Contractility at Single‐cell Resolution.” EMBO Reports 20(12):1–14.
Equipment used: Photomanipulation system UGA-42 Firefly with pulsed 14mW UV laser DPSL-355/14 (UGA-42 Caliburn) - Pons-Vizcarra, Maria, Julia Kurps, Bassam Tawfik, Jakob B. Sørensen, Jan R. T. van Weering, and Matthijs Verhage. 2019. “Erratum: MUNC18-1 Regulates the Submembrane F-Actin Network, Independently of Syntaxin1 Targeting, via Hydrophobicity in β-Sheet 10 (Journal of Cell Science (2019) 132 (Jcs234674) DOI: 10.1242/Jcs.234674).” Journal of Cell Science 132(23).
Equipment used: Flash lamp system JML-C2 - Shindou, Tomomi, Mayumi Shindou, Sakurako Watanabe, and Jeffery Wickens. 2019. “A Silent Eligibility Trace Enables Dopamine-Dependent Synaptic Plasticity for Reinforcement Learning in the Mouse Striatum.” European Journal of Neuroscience 49(5):726–36.
Equipment used: Flash lamp system JML-C2 - Wilson, Calum, Matthew D. Lee, Helen R. Heathcote, Xun Zhang, Charlotte Buckley, John M. Girkin, Christopher D. Saunter, and John G. McCarron. “Mitochondrial ATP Production Provides Long-Range Control of Endothelial Inositol Trisphosphate– Evoked Calcium Signaling.” Journal of Biological Chemistry 294(3):737–58. doi: 10.1074/jbc.RA118.005913.
Equipment used: Flash lamp system JML-C2 - Wilson, Calum, Xun Zhang, Charlotte Buckley, Helen R. Heathcote, Matthew D. Lee, and John G. McCarron. 2019. “Increased Vascular Contractility in Hypertension Results from Impaired Endothelial Calcium Signaling.” Hypertension. 74(5):1200–1214.
Equipment used: Flash lamp system JML-C2 - Zhang, Xun, Matthew D. Lee, Calum Wilson, and John G. McCarron. “Hydrogen Peroxide Depolarizes Mitochondria and Inhibits IP3-Evoked Ca2+ Release in the Endothelium of Intact Arteries.” Cell Calcium 84(October):102108. doi: 10.1016/j.ceca.2019.102108.
Equipment used: Flash lamp system JML-C2
2018
- Huang, Chien Chang, Tai Yu Chiu, Tzu Ying Lee, Hsin Jui Hsieh, Chung Chih Lin, and Lung Sen Kao. 2018. “Soluble α-Synuclein Facilitates Priming and Fusion by Releasing Ca2+ from the Thapsigargin-Sensitive Ca2+ Pool in PC12 Cells.” Journal of Cell Science 131(23).
Equipment used: Flash lamp system JML-C2 - Palma-Cerda, Francisco, George Papageorgiou, Boris Barbour, Céline Auger, and David Ogden. 2018. “Photolysis of a Caged, Fast-Equilibrating Glutamate Receptor Antagonist, MNI-Caged γ-D-Glutamyl-Glycine, to Investigate Transmitter Dynamics and Receptor Properties at Glutamatergic Synapses.” Frontiers in Cellular Neuroscience 12(December):1–12.
Equipment used: Flash lamp system JML-C2 - Przibilla, Julia, Sandeep Dembla, Oleksandr Rizun, Annette Lis, Martin Jung, Johannes Oberwinkler, Andreas Beck, and Stephan E. Philipp. 2018. “Ca2+-Dependent Regulation and Binding of Calmodulin to Multiple Sites of Transient Receptor Potential Melastatin 3 (TRPM3) Ion Channels.” Cell Calcium 73:40–52.
Equipment used: Flash lamp system JML-C2 - Windler, F., W. Bönigk, H. G. Körschen, E. Grahn, T. Strünker, R. Seifert, and U. B. Kaupp. 2018. “The Solute Carrier SLC9C1 Is a Na+/H+-Exchanger Gated by an S4-Type Voltage-Sensor and Cyclic-Nucleotide Binding.” Nature Communications 9(1):1–13.
Equipment used: Flash lamp system JML-C2 - Xian, Wenying, Xin Hui, Qinghai Tian, Hongmei Wang, Alessandra Moretti, Karl Ludwig Laugwitz, Veit Flockerzi, Sandra Ruppenthal, and Peter Lipp. 2018. “Aberrant Deactivation-Induced Gain of Function in TRPM4 Mutant Is Associated with Human Cardiac Conduction Block.” Cell Reports 24(3):724–31.
Equipment used: Flash lamp system JML-C2 - Yapo, Cȩdric, Anu G. Nair, Jeanette Hellgren Kotaleski, Pierre Vincent, and Liliana R. V. Castro. 2018. “Switch-like PKA Responses in the Nucleus of Striatal Neurons.” Journal of Cell Science 131(14).
Equipment used: Spot illumination module (AiWon) with UV LED System UVILED
2017
- Fernandez, Laura M. J., Chiara Pellegrini, Gil Vantomme, Elidie Béard, Anita Lüthi, and Simone Astori. 2017. “Cortical Afferents onto the Nucleus Reticularis Thalami Promote Plasticity of Low-Threshold Excitability through GluN2C-NMDARs.” Scientific Reports 7(1):1–13.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473nm - Houy, Sébastien, Alexander J. Groffen, Iwona Ziomkiewicz, Matthijs Verhage, Paulo S. Pinheiro, and Jakob Balslev Sørensen. 2017. “Doc2B Acts as a Calcium Sensor for Vesicle Priming Requiring Synaptotagmin-1, Munc13-2 and SNAREs.” ELife 6:1–29.
Equipment used: Flash lamp system JML-C2 - Hui, Xin, Benjamin Sauer, Lars Kaestner, Karsten Kruse, and Peter Lipp. 2017. “PKCα Diffusion and Translocation Are Independent of an Intact Cytoskeleton.” Scientific Reports 7(1):1–11.
Equipment used: Flash lamp system JML-C2 - Kirchner, Matthew K., Robert C. Foehring, Lie Wang, Giri Kumar Chandaka, Joseph C. Callaway, and William E. Armstrong. 2017. “Phosphatidylinositol 4,5-Bisphosphate (PIP2) Modulates Afterhyperpolarizations in Oxytocin Neurons of the Supraoptic Nucleus.” Journal of Physiology 595(14):4927–46.
Equipment used: Flash lamp system JML-C2 - Kovács, Adrienn and Balázs Pál. 2017. “Astrocyte-Dependent Slow Inward Currents (SICs) Participate in Neuromodulatory Mechanisms in the Pedunculopontine Nucleus (PPN).” Frontiers in Cellular Neuroscience 11(February):1–16.
Equipment used: Flash lamp system JML-C2 - Taylor, Emily J. A., Evangelia Pantazaka, Kathryn L. Shelley, and Colin W. Taylor. 2017. “Prostaglandin E2 Inhibits Histamine-Evoked Ca21 Release in Human Aortic Smooth Muscle Cells through Hyperactive Camp Signaling Junctions and Protein Kinase A.” Molecular Pharmacology 92(5):533–45.
Equipment used: Flash lamp system JML-C2 - Walter, Alexander M., Rainer Müller, Bassam Tawfik, Keimpe Db Wierda, Paulo S. Pinheiro, André Nadler, Anthony W. McCarthy, Iwona Ziomkiewicz, Martin Kruse, Gregor Reither, Jens Rettig, Martin Lehmann, Volker Haucke, Bertil Hille, Carsten Schultz, and Jakob Balslev Sørensen. 2017. “Phosphatidylinositol 4,5-Bisphosphate Optical Uncaging Potentiates Exocytosis.” ELife 6(2017):e30203.
Equipment used: Flash lamp system JML-C2
2016
- Dvorzhak, Anton, Tatyana Vagner, Knut Kirmse, and Rosemarie Grantyn. 2016. “Functional Indicators of Glutamate Transport in Single Striatal Astrocytes and the Influence of Kir4.1 in Normal and Huntington Mice.” The Journal of Neuroscience 36(18):4959–75. doi: 10.1523/JNEUROSCI.0316-16.2016.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly). - Hou, Panpan, Feng Xiao, Haowen Liu, Ming Yuchi, Guohui Zhang, Ying Wu, Wei Wang, Wenping Zeng, Mingyue Ding, Jianming Cui, Zhengxing Wu, Lu Yang Wang, and Jiuping Ding. 2016. “Extrapolating Microdomain Ca2+ Dynamics Using BK Channels as a Ca2+ Sensor.” Scientific Reports 6:1–11.
Equipment used: Flash lamp system JML-C2 - Kreye, Jakob, Nina K. Wenke, Mariya Chayka, Jonas Leubner, Rajagopal Murugan, Nikolaus Maier, Betty Jurek, Lam Thanh Ly, Doreen Brandl, Benjamin R. Rost, Alexander Stumpf, Paulina Schulz, Helena Radbruch, Anja E. Hauser, Florence Pache, Andreas Meisel, Lutz Harms, Friedemann Paul, Ulrich Dirnagl, Craig Garner, Dietmar Schmitz, Hedda Wardemann, and Harald Prüss. 2016. “Human Cerebrospinal Fluid Monoclonal N-Methyl-D-Aspartate Receptor Autoantibodies Are Sufficient for Encephalitis Pathogenesis.” Brain 139(10):2641–52.
Equipment used: Spot illumination module (AiWon) with pulsed 30mW UV laser DPSS-355 - Munch, Anders S., Girish H. Kedar, Jan R. T. van Weering, Sonia Vazquez-Sanchez, Enqi He, Timon André, Thimo Braun, Thomas H. Söllner, Matthijs Verhage, and Jakob B. Sørensen. 2016. “Extension of Helix 12 in Munc18-1 Induces Vesicle Priming.” Journal of Neuroscience 36(26):6881–91.
Equipment used: Flash lamp system JML-C2 - Pietra, Gianluca, Michele Dibattista, Anna Menini, Johannes Reisert, and Anna Boccaccio. 2016. “The Ca2+-Activated Cl- Channel TMEM16B Regulates Action Potential Firing and Axonal Targeting in Olfactory Sensory Neurons.” Journal of General Physiology 148(4):1–19.
Equipment used: Flash lamp system JML-C2 - Toft-Bertelsen, Trine Lisberg, Iwona Ziomkiewicz, Sebastien Houy, Paulo S. Pinheiro, and Jakob B. Sørensen. 2016. “Regulation of Ca2+ Channels by SNAP-25 via Recruitment of Syntaxin-1 from Plasma Membrane Clusters.” Molecular Biology of the Cell 27(21):3329–41.
Equipment used: Flash lamp system JML-C2 - Wilson, Calum, Christopher D. Saunter, John M. Girkin, and John G. McCarron. 2016. “Clusters of Specialized Detector Cells Provide Sensitive and High Fidelity Receptor Signaling in the Intact Endothelium.” FASEB Journal 30(5):2000–2013.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with pulsed 30mW UV laser DPSS-355/30
2015
- Bedner, Peter, Alexander Dupper, Kerstin Hüttmann, Julia Müller, Michel K. Herde, Pavel Dublin, Tushar Deshpande, Johannes Schramm, Ute Häussler, Carola A. Haas, Christian Henneberger, Martin Theis, and Christian Steinhäuser. 2015. “Astrocyte Uncoupling as a Cause of Human Temporal Lobe Epilepsy.” Brain 138(5):1208–22.
Equipment used: Flash lamp system JML-C2 - Böhm, Claudia, Maria Pangalos, Dietmar Schmitz, and Jochen Winterer. 2015. “Serotonin Attenuates Feedback Excitation onto O-LM Interneurons.” Cerebral Cortex 25(11):4572–83.
Equipment used: Spot illumination module with pulsed UV laser DPSL-355 ( AiWon Caliburn) - Capel, Rebecca A., Emma L. Bolton, Wee K. Lin, Daniel Aston, Yanwen Wang, Wei Liu, Xin Wang, Rebecca Ann B. Burton, Duncan Bloor-Young, Kai Ting Shade, Margarida Ruas, John Parrington, Grant C. Churchill, Ming Lei, Antony Galione, and Derek A. Terrar. 2015. “Two-Pore Channels (TPC2s) and Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) at Lysosomal-Sarcoplasmic Reticular Junctions Contribute to Acute and Chronic β-Adrenoceptor Signaling in the Heart.” Journal of Biological Chemistry 290(50):30087–98.
Equipment used: Flash lamp system JML-C2 - Chen, Minghui, Matthew J. Van Hook, and Wallace B. Thoreson. 2015. “Ca2+ Diffusion through Endoplasmic Reticulum Supports Elevated Intraterminal Ca2+ Levels Needed to Sustain Synaptic Release from Rods in Darkness.” Journal of Neuroscience 35(32):11364–73.
Equipment used: Flash lamp system JML-C2 - Fechner, Sylvia, Luis Alvarez, Wolfgang Bönigk, Astrid Müller, Thomas K. Berger, Rene Pascal, Christian Trötschel, Ansgar Poetsch, Gabriel Stölting, Kellee R. Siegfried, Elisabeth Kremmer, Reinhard Seifert, and U. Benjamin Kaupp. 2015. “A K+-Selective CNG Channel Orchestrates Ca2+ Signalling in Zebrafish Sperm.” ELife 4(DECEMBER2015):1–25.
Equipment used: Flash lamp system JML-C2 - Gómez-Gonzalo, Marta, Marta Navarrete, Gertrudis Perea, Ana Covelo, Mario Martín-Fernández, Ryuichi Shigemoto, Rafael Luján, and Alfonso Araque. 2015. “Endocannabinoids Induce Lateral Long-Term Potentiation of Transmitter Release by Stimulation of Gliotransmission.” Cerebral Cortex 25(10):3699–3712.
Equipment used: Flash lamp system JML-C2 - Kandasamy, Kathirvel, Rachel Escue, Jayeeta Manna, Adebowale Adebiyi, and Kaushik Parthasarathi. 2015. “Changes in Endothelial Connexin 43 Expression Inversely Correlate with Microvessel Permeability and VE-Cadherin Expression in Endotoxin-Challenged Lungs.” American Journal of Physiology – Lung Cellular and Molecular Physiology 309(6):L584–92.
Equipment used: Flash lamp system JML-C2 - Kochubey, Olexiy, and Ralf Schneggenburger. 2015. “Ca 2+ Uncaging in Nerve Terminals: A Three-Point Calibration Procedure.” Cold Spring Harbor Protocols 2015(8):pdb.prot087650. doi: 10.1101/pdb.prot087650.
Equipment used: Flash lamp system SP-20 - Körber, Christoph, Heinz Horstmann, Varun Venkataramani, Frank Herrmannsdörfer, Thomas Kremer, Michaela Kaiser, Darius B. Schwenger, Saheeb Ahmed, Camin Dean, Thomas Dresbach, and Thomas Kuner. 2015. “Modulation of Presynaptic Release Probability by the Vertebrate-Specific Protein Mover.” Neuron 87(3):521–33.
Equipment used: Flash lamp system JML-C2 - Seifert, Reinhard, Melanie Flick, Wolfgang Bönigk, Luis Alvarez, Christian Trötschel, Ansgar Poetsch, Astrid Müller, Normann Goodwin, Patric Pelzer, Nachiket D. Kashikar, Elisabeth Kremmer, Jan Jikeli, Bernd Timmermann, Heiner Kuhl, Dmitry Fridman, Florian Windler, U. Benjamin Kaupp, and Timo Strünker. 2015. “The CatSper Channel Controls Chemosensation in Sea Urchin Sperm.” The EMBO Journal 34(3):379–92.
Equipment used: Flash lamp system JML-C2 - Staiger, Jochen F., Ingo Bojak, Stéphanie Miceli, and Dirk Schubert. 2015. “A Gradual Depth-Dependent Change in Connectivity Features of Supragranular Pyramidal Cells in Rat Barrel Cortex.” Brain Structure and Function 220(3):1317–37.
Equipment used: Flash lamp system JML-C2
2014
- Neef, Jakob, Sangyong Jung, Aaron B. Wong, Kirsten Reuter, Tina Pangrsic, Rituparna Chakrabarti, Sebastian Kügler, Christine Lenz, Régis Nouvian, Rebecca M. Boumil, Wayne N. Frankel, Carolin Wichmann, and Tobias Moser. 2014. “Modes and Regulation of Endocytic Membrane Retrieval in Mouse Auditory Hair Cells.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 34(3):705–16.
Equipment used: Flash lamp system JML-C2 - Kleinhans, Christian, Karl W. Kafitz, and Christine R. Rose. 2014. “Multi-Photon Intracellular Sodium Imaging Combined with UV-Mediated Focal Uncaging of Glutamate in CA1 Pyramidal Neurons.” Journal of Visualized Experiments (92):1–11. doi: 10.3791/52038.
Equipment used:Photomanipulation system UGA-42 with UV laser DPSS 355/30 - Walter, Alexander M., Julia Kurps, Heidi de Wit, Susanne Schöning, Trine L. Toft-Bertelsen, Juliane Lauks, Iwona Ziomkiewicz, Annita N. Weiss, Alexander Schulz, Gabriele Fischer von Mollard, Matthijs Verhage, and Jakob B. Sørensen. 2014. “The SNARE Protein Vti1a Functions in Dense-Core Vesicle Biogenesis.” The EMBO Journal 33(15):1681–97.
Equipment used: Flash lamp system JML-C2 - Westphalen, Kristin, Galina A. Gusarova, Mohammad N. Islam, Manikandan Subramanian, Taylor S. Cohen, Alice S. Prince, and Jahar Bhattacharya. 2014. “Sessile Alveolar Macrophages Communicate with Alveolar Epithelium to Modulate Immunity.” Nature 506(7489):503–6.
Equipment used: Flash lamp system JML-C2 - Wong, Aaron B., Mark A. Rutherford, Mantas Gabrielaitis, Tina Pangršič, Fabian Göttfert, Thomas Frank, Susann Michanski, Stefan Hell, Fred Wolf, Carolin Wichmann, and Tobias Moser. 2014. “Developmental Refinement of Hair Cell Synapses Tightens the Coupling of Ca 2 + Influx to Exocytosis.” The EMBO Journal 33(3):n/a-n/a.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/100
2013
- Beed, Prateep, Anja Gundlfinger, Sophie Schneiderbauer, Jie Song, Claudia Böhm, Andrea Burgalossi, Michael Brecht, Imre Vida, and Dietmar Schmitz. 2013. “Inhibitory Gradient along the Dorsoventral Axis in the Medial Entorhinal Cortex.” Neuron 79(6):1197–1207.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/1000 - Hugo, Sandra, Ekta Dembla, Mahantappa Halimani, Ulf Matti, Jens Rettig, and Ute Becherer. 2013. “Deciphering Dead-End Docking of Large Dense Core Vesicles in Bovine Chromaffin Cells.” Journal of Neuroscience 33(43):17123–37.
Equipment used: Flash lamp system JML-C2 - López-Sanjurjo, Cristina I., Stephen C. Tovey, David L. Prole, and Colin W. Taylor. 2013. “Lysosomes Shape Ins(1,4,5)P3-Evoked Ca2+ Signals by Selectively Sequestering Ca2+ Released from the Endoplasmic Reticulum.” Journal of Cell Science 126(Pt 1):289–300.
Equipment used: Flash lamp system JML-C2 - Miyazaki, K. and W. N. Ross. 2013. “Ca2+ Sparks and Puffs Are Generated and Interact in Rat Hippocampal CA1 Pyramidal Neuron Dendrites.” Journal of Neuroscience 33(45):17777–88.
Equipment used: Spot illumination module (AiWon) with UV LED System UVILED - Tovey, Stephen C. and Colin W. Taylor. 2013. “Cyclic AMP Directs Inositol (1,4,5)-Trisphosphate-Evoked Ca2+ Signalling to Different Intracellular Ca2+ Stores.” Journal of Cell Science 126(Pt 10):2305–13.
Equipment used: Flash lamp system JML-C2 - Whitesell, Jennifer D., Kyle a Sorensen, Brooke C. Jarvie, Shane T. Hentges, and Nathan E. Schoppa. 2013. “Interglomerular Lateral Inhibition Targeted on External Tufted Cells in the Olfactory Bulb.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 33(4):1552–63. Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with pulsed 30mW UV laser DPSS-355/30
2012
- Anastasiades, Paul G. and Simon J. B. Butt. 2012. “A Role for Silent Synapses in the Development of the Pathway from Layer 2/3 to 5 Pyramidal Cells in the Neocortex.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 32(38):13085–99.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with pulsed 30mW UV laser DPSS-355/30 - Chalmers, Susan, Stuart T. Caldwell, Caroline Quin, Tracy A. Prime, Andrew M. James, Andrew G. Cairns, Michael P. Murphy, John G. McCarron, and Richard C. Hartley. 2012. “Selective Uncoupling of Individual Mitochondria within a Cell Using a Mitochondria-Targeted Photoactivated Protonophore.” Journal of the American Chemical Society 134(2):758–61.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with pulsed 30mW UV laser DPSS-355/30 - Couchman, Kiri, Benedikt Grothe, and Felix Felmy. 2012. “Functional Localization of Neurotransmitter Receptors and Synaptic Inputs to Mature Neurons of the Medial Superior Olive.” Journal of Neurophysiology 107(4):1186–98.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/1000 - Cui, Jiaxi, Radu A. Gropeanu, David R. Stevens, Jens Rettig, and Aránzazu Del Campo. 2012 “New Photolabile BAPTA-Based Ca 2+ Cages with Improved Photorelease.” Journal of the American Chemical Society 134(18):7733–40. doi: 10.1021/ja2115184.
Equipment used: Flash lamp system (JML-C2) - Joshi, Khashti Ballabh, Andreas Vlachos, Vera Mikat, Thomas Deller, and Alexander Heckel. 2012. “Light-Activatable Molecular Beacons with a Caged Loop Sequence.” Commun. 48(22):2746–48. doi: 10.1039/C2CC16654B.
Equipment used: Spot illumination module (AiWon) with UV LED System UVILED - MacMillan, D., C. Kennedy, and J. G. McCarron. 2012. “ATP Inhibits Ins(1,4,5)P3-Evoked Ca2+ Release in Smooth Muscle via P2Y1 Receptors.” Journal of Cell Science 125(21):5151–58.
Equipment used: Flash lamp system (JML-C2) - Olson, Marnie L., Mairi E. Sandison, Susan Chalmers, and John G. McCarron. 2012. “Microdomains of Muscarinic Acetylcholine and Ins(1,4,5) P 3 Receptors Create ‘Ins(1,4,5) P 3 Junctions’ and Sites of Ca 2+ Wave Initiation in Smooth Muscle.” Journal of Cell Science 125(22):5315–28. Equipment used: Flash lamp system (JML-C2)
2011
- Bell, Karen a, Hoon Shim, Ching-Kang Chen, and a Rory McQuiston. 2011. “Nicotinic Excitatory Postsynaptic Potentials in Hippocampal CA1 Interneurons Are Predominantly Mediated by Nicotinic Receptors That Contain Α4 and Β2 Subunits.” Neuropharmacology 61(8):1379–88. doi: 10.1016/j.neuropharm.2011.08.024.
Equipment used: Flash lamp system (JML-C2) - Billig, Gwendolyn M., Balázs Pál, Pawel Fidzinski, and Thomas J. Jentsch. 2011. “Ca2+-Activated Cl− Currents Are Dispensable for Olfaction.” Nature Neuroscience 14(6):763–69.
Equipment used: Flash lamp system JML-C2 - Jerome, Jason and Detlef H. Heck. 2011. “The Age of Enlightenment: Evolving Opportunities in Brain Research through Optical Manipulation of Neuronal Activity.” Frontiers in Systems Neuroscience 5(December):95.
Equipment used: Flash lamp system (JML-C2) - Manita, Satoshi, Kenichi Miyazaki, and William N. Ross. 2011. “Synaptically Activated Ca2+ Waves and NMDA Spikes Locally Suppress Voltage-Dependent Ca2+ Signalling in Rat Pyramidal Cell Dendrites.” The Journal of Physiology 589(Pt 20):4903–20.
Equipment used: Spot illumination module (AiWon) with UV LED System UVILED - Nouvian, Régis, Jakob Neef, Anna V Bulankina, Ellen Reisinger, Tina Pangršič, Thomas Frank, Stefan Sikorra, Nils Brose, Thomas Binz, and Tobias Moser. 2011. “Exocytosis at the Hair Cell Ribbon Synapse Apparently Operates without Neuronal SNARE Proteins.” Nature Neuroscience 14(4):411–13.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/1000 - Thurley, Kevin, Ian F. Smith, Stephen C. Tovey, Colin W. Taylor, Ian Parker, and Martin Falcke. 2011. “Timescales of IP(3)-Evoked Ca(2+) Spikes Emerge from Ca(2+) Puffs Only at the Cellular Level.” Biophysical Journal 101(11):2638–44.
Equipment used: Flash lamp system (JML-C2 - Umeda, Nobuhiro, Tasuku Ueno, Christopher Pohlmeyer, Tetsuo Nagano, and Takanari Inoue. 2011. “A Photocleavable Rapamycin Conjugate for Spatiotemporal Control of Small GTPase Activity.” Journal of the American Chemical Society 133(1):12–14.
Equipment used: Spot illumination module (AiWon) with UV LED System UVILED - Wiegand, Hauke F., Prateep Beed, Michael H. K. Bendels, Christian Leibold, Dietmar Schmitz, and Friedrich W. Johenning. 2011. “Complementary Sensory and Associative Microcircuitry in Primary Olfactory Cortex.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 31(34):12149–58.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/1000
2010
- Beed, Prateep, Michael H. K. Bendels, Hauke F. Wiegand, Christian Leibold, Friedrich W. Johenning, and Dietmar Schmitz. 2010. “Analysis of Excitatory Microcircuitry in the Medial Entorhinal Cortex Reveals Cell-Type-Specific Differences.” Neuron 68(6):1059–66.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/1000 - Chepurny, Oleg G., Grant G. Kelley, Igor Dzhura, Colin A. Leech, Michael W. Roe, Elvira Dzhura, Xiangquan Li, Frank Schwede, Hans-G. Genieser, and George G. Holz. 2010. “PKA-Dependent Potentiation of Glucose-Stimulated Insulin Secretion by Epac Activator 8-PCPT-2’-O-Me-CAMP-AM in Human Islets of Langerhans.” American Journal of Physiology. Endocrinology and Metabolism 298(3):E622-33.
Equipment used: Flash lamp system JML-C2 - Dzhura, Igor, Oleg G. Chepurny, Grant G. Kelley, Colin a Leech, Michael W. Roe, Elvira Dzhura, Parisa Afshari, Sundeep Malik, Michael J. Rindler, Xin Xu, Youming Lu, Alan V Smrcka, and George G. Holz. 2010. “Epac2-Dependent Mobilization of Intracellular Ca2+ by Glucagon-like Peptide-1 Receptor Agonist Exendin-4 Is Disrupted in β-Cells of Phospholipase C-ε Knockout Mice.” The Journal of Physiology 588(Pt 24):4871–89.
Equipment used: Flash lamp system JML-C2 - Frank, Thomas, Mark a Rutherford, Nicola Strenzke, Andreas Neef, Tina Pangršič, Darina Khimich, Anna Fejtova, Anna Fetjova, Eckart D. Gundelfinger, M. Charles Liberman, Benjamin Harke, Keith E. Bryan, Amy Lee, Alexander Egner, Dietmar Riedel, and Tobias Moser. 2010. “Bassoon and the Synaptic Ribbon Organize Ca2+ Channels and Vesicles to Add Release Sites and Promote Refilling.” Neuron 68(4):724–38.
Equipment used: Flash lamp system (JML-C2) - Guerrero, Adan, Takuya Nishigaki, Jorge Carneiro, Yoshiro Tatsu, Christopher D. Wood, and Alberto Darszon. 2010. “Tuning Sperm Chemotaxis by Calcium Burst Timing.” Developmental Biology 344(1):52–65. doi: 10.1016/j.ydbio.2010.04.013.
Equipment used: high power short wave UV illumination system UVICO (UVICO-2) - Kukley, Maria, Akiko Nishiyama, and Dirk Dietrich. 2010. “The Fate of Synaptic Input to NG2 Glial Cells: Neurons Specifically Downregulate Transmitter Release onto Differentiating Oligodendroglial Cells.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 30(24):8320–31.
Equipment used: Flash lamp system JML-C2 - Lee, Seunghoon, Kyongmin Kim, and Z. Jimmy Zhou. 2010. “Role of ACh-GABA Cotransmission in Detecting Image Motion and Motion Direction.” Neuron 68(6):1159–72.
Equipment used: Spot illumination module (AiWon) with pulsed 30mW UV laser DPSS-355/30 - MacMillan, D. and J. G. McCarron. 2010. “The Phospholipase C Inhibitor U-73122 Inhibits Ca2+ Release from the Intracellular Sarcoplasmic Reticulum Ca2+ Store by Inhibiting Ca2+ Pumps in Smooth Muscle.” British Journal of Pharmacology 160(6):1295–1301.
Equipment used: Flash lamp system (JML-C2) - McCarron, John G., Susan Chalmers, Debbi MacMillan, and Marnie L. Olson. 2010. “Agonist-Evoked Ca2+ Wave Progression Requires Ca2+ and IP3.” Journal of Cellular Physiology 224(2):334–44. Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with pulsed 30mW UV laser DPSS-355/30
- Olson, M. L., S. Chalmers, and J. G. McCarron. 2010. “Mitochondrial Ca2+ Uptake Increases Ca2+ Release from Inositol 1,4,5-Trisphosphate Receptor Clusters in Smooth Muscle Cells.” J Biol Chem 285(3):2040–50.
Equipment used: Flash lamp system (JML-C2) - Walter, Alexander M., Katrin Wiederhold, Dieter Bruns, Dirk Fasshauer, and Jakob B. Sørensen. 2010. “Synaptobrevin N-Terminally Bound to Syntaxin-SNAP-25 Defines the Primed Vesicle State in Regulated Exocytosis.” Journal of Cell Biology 188(3):401–13.
Equipment used: Flash lamp system (JML-C2)
2009
- Bönigk, Wolfgang, Astrid Loogen, Reinhard Seifert, Nachiket Kashikar, Clementine Klemm, Eberhard Krause, Volker Hagen, Elisabeth Kremmer, Timo Strünker, and U. Benjamin Kaupp. 2009. “An Atypical CNG Channel Activated by a Single CGMP Molecule Controls Sperm Chemotaxis.” Science Signaling 2(94):ra68.
Equipment used: Double pulse flash lamp system DP-10 - Kochubey, Olexiy, Yunyun Han, and Ralf Schneggenburger. 2009. “Developmental Regulation of the Intracellular Ca2+ Sensitivity of Vesicle Fusion and Ca2+-Secretion Coupling at the Rat Calyx of Held.” The Journal of Physiology 587(Pt 12):3009–23.
Equipment used: Flash lamp system SP-20 - Pifferi, Simone, Michele Dibattista, Claudia Sagheddu, Anna Boccaccio, Ahmed Al Qteishat, Filippo Ghirardi, Roberto Tirindelli, and Anna Menini. 2009. “Calcium-Activated Chloride Currents in Olfactory Sensory Neurons from Mice Lacking Bestrophin-2.” The Journal of Physiology 587(Pt 17):4265–79.
Equipment used: Flash lamp system JML-C2 - Scimemi, Annalisa, Hua Tian, and Jeffrey S. Diamond. 2009. “Neuronal Transporters Regulate Glutamate Clearance, NMDA Receptor Activation, and Synaptic Plasticity in the Hippocampus.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 29(46):14581–95. Equipment used: Flash lamp system FlashMic (JML-C2)
- Shaikh, Tanvir R., David Barnard, Xing Meng, and Terence Wagenknecht. 2009. “Implementation of a Flash-Photolysis System for Time-Resolved Cryo-Electron Microscopy.” Journal of Structural Biology 165(3):184–89.
Equipment used: Flash lamp system JML-C1 (JML-C2) - Trigo, Federico F., George Papageorgiou, John E. T. Corrie, and David Ogden. 2009. “Laser Photolysis of DPNI-GABA, a Tool for Investigating the Properties and Distribution of GABA Receptors and for Silencing Neurons in Situ.” Journal of Neuroscience Methods 181(2):159–69.
Equipment used: Flash lamp system (JML-C2)
2008
- Bendels, Michael H. K., Prateep Beed, Christian Leibold, Dietmar Schmitz, and Friedrich W. Johenning. 2008. “A Novel Control Software That Improves the Experimental Workflow of Scanning Photostimulation Experiments.” Journal of Neuroscience Methods 175(1):44–57.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/1000 - Cai, Haijiang, Kerstin Reim, Frederique Varoqueaux, Sompol Tapechum, Kerstin Hill, Jakob B. Sørensen, Nils Brose, and Robert H. Chow. 2008. “Complexin II Plays a Positive Role in Ca2+-Triggered Exocytosis by Facilitating Vesicle Priming.” Proceedings of the National Academy of Sciences of the United States of America 105(49):19538–43.
Equipment used: Flash lamp system XF-10 (JML-C2) - Hagenston, Anna M., John S. Fitzpatrick, and Mark F. Yeckel. 2008. “MGluR-Mediated Calcium Waves That Invade the Soma Regulate Firing in Layer V Medial Prefrontal Cortical Pyramidal Neurons.” Cerebral Cortex (New York, N.Y. : 1991) 18(2):407–23.
Equipment used: Spot illumination module (AiWon) - Karatekin, Erdem, Viet Samuel Tran, Sébastien Huet, Isabelle Fanget, Sophie Cribier, and Jean-Pierre Henry. 2008. “A 20-Nm Step toward the Cell Membrane Preceding Exocytosis May Correspond to Docking of Tethered Granules.” Biophysical Journal 94(7):2891–2905.
Equipment used: Flash lamp system JML-C2 - Knoll, Helmut. 2008. “Effects of Unidirectional and Mutual Interactions between Microstructures and Azo Dyes as ‘Kinetic’ Probe Molecules on Cis→trans Isomerization Rate Constants in Aqueous P85 and F88 Triblock Copolymer Solutions.” International Journal of Chemical Kinetics 40(2):59–65. doi: 10.1002/kin.20298.
Equipment used: flash lamp system
2007
- Boccaccio, Anna and Anna Menini. 2007. “Temporal Development of Cyclic Nucleotide-Gated and Ca2+ -Activated Cl- Currents in Isolated Mouse Olfactory Sensory Neurons.” Journal of Neurophysiology 98(1):153–60.
Equipment used: Flash lamp system JML-C2 - Hong, Min and William N. Ross. 2007. “Priming of Intracellular Calcium Stores in Rat CA1 Pyramidal Neurons.” The Journal of Physiology 584(Pt 1):75–87.
Equipment used: Spot illumination module (AiWon) with UV LED System UVILED - Jin, Yunju, Sang Jeong Kim, Jun Kim, Paul F. Worley, and David J. Linden. 2007. “Long-Term Depression of MGluR1 Signaling.” Neuron 55(2):277–87.
Equipment used: Spot illumination module (AiWon) with pulsed 1W UV laser DPSL-355/1000 - Kurtz, R. 2007. “Direction-Selective Adaptation in Fly Visual Motion-Sensitive Neurons Is Generated by an Intrinsic Conductance-Based Mechanism.” Neuroscience 146(2):573–83.
Equipment used: Flash lamp system JML-C2 - Korogod, Natalya, Xuelin Lou, and Ralf Schneggenburger. 2007. “Posttetanic Potentiation Critically Depends on an Enhanced Ca(2+) Sensitivity of Vesicle Fusion Mediated by Presynaptic PKC.” Proceedings of the National Academy of Sciences of the United States of America 104(40):15923–28. Equipment used: Flash lamp system SP-20
- Sobie, Eric A., Joseph P. Y. Kao, and W. J. Lederer. 2007. “Novel Approach to Real-Time Flash Photolysis and Confocal [Ca2+] Imaging.” Pflügers Archiv : European Journal of Physiology 454(4):663–73.
Equipment used: Spot illumination module (AiWon) - Tran, Viet Samuel, Sébastien Huet, Isabelle Fanget, Sophie Cribier, Jean-Pierre Henry, and Erdem Karatekin. 2007. “Characterization of Sequential Exocytosis in a Human Neuroendocrine Cell Line Using Evanescent Wave Microscopy and ‘Virtual Trajectory’ Analysis.” European Biophysics Journal : EBJ 37(1):55–69.
Equipment used: Flash lamp system JML-C2 - Wadel, Kristian, Erwin Neher, and Takeshi Sakaba. 2007. “The Coupling between Synaptic Vesicles and Ca2+ Channels Determines Fast Neurotransmitter Release.” Neuron 53(4):563–75. Equipment used: Flash lamp system (JML-C2) with light guide adapter LGA
- Wölfel, Markus, Xuelin Lou, and Ralf Schneggenburger. 2007. “A Mechanism Intrinsic to the Vesicle Fusion Machinery Determines Fast and Slow Transmitter Release at a Large CNS Synapse.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 27(12):3198–3210. Equipment used: Flash lamp system SP-20
2006
- Boccaccio, Anna, Laura Lagostena, Volker Hagen, and Anna Menini. 2006. “Fast Adaptation in Mouse Olfactory Sensory Neurons Does Not Require the Activity of Phosphodiesterase.” The Journal of General Physiology 128(2):171–84.
Equipment used: Flash lamp system JML-C2 - Canepari, Marco and David Ogden. 2006. “Kinetic, Pharmacological and Activity-Dependent Separation of Two Ca2+ Signalling Pathways Mediated by Type 1 Metabotropic Glutamate Receptors in Rat Purkinje Neurones.” The Journal of Physiology 573(Pt 1):65–82.
Equipment used: Flash lamp system JML-C2 - Höpflinger, Marion Christine, Olena Andruchova, Oleg Andruchov, Herbert Grassberger, and Stefan Galler. 2006. “Effect of PH on the Rate of Myosin Head Detachment in Molluscan Catch Muscle: Are Myosin Heads Involved in the Catch State?” The Journal of Experimental Biology 209(Pt 4):668–76.
Equipment used: Flash lamp system (JML-C2) - Ichimura, Hideo, Kaushik Parthasarathi, Jens Lindert, and Jahar Bhattacharya. 2006. “Lung Surfactant Secretion by Interalveolar Ca 2+ Signaling.” American Journal of Physiology-Lung Cellular and Molecular Physiology 291(4):L596–601.
Equipment used: Flash lamp system JML-C2 - Nache, Vasilica, Jana Kusch, Volker Hagen, and Klaus Benndorf. 2006. “Gating of Cyclic Nucleotide-Gated (CNGA1) Channels by CGMP Jumps and Depolarizing Voltage Steps.” Biophysical Journal 90(9):3146–54.
Equipment used: Flash lamp system JML-C2 - Parthasarathi, Kaushik. 2006. “Connexin 43 Mediates Spread of Ca2+ -Dependent Proinflammatory Responses in Lung Capillaries.” Journal of Clinical Investigation 116(8):2193–2200.
Equipment used: Flash lamp system JML-C2 - Sperandio, Markus, John E. Pickard, Sunil Unnikrishnan, Scott T. Acton, and Klaus Ley. 2006. “Analysis of Leukocyte Rolling in Vivo and in Vitro.” Methods in Enzymology 416:346–71. Equipment used: Flash lamp system (JML-C2)
- Straub, Stephen V, Adrian D. Bonev, M. Keith Wilkerson, and Mark T. Nelson. 2006. “Dynamic Inositol Trisphosphate-Mediated Calcium Signals within Astrocytic Endfeet Underlie Vasodilation of Cerebral Arterioles.” The Journal of General Physiology 128(6):659–69.
Equipment used: Flash lamp system (JML-C2)
2005
- Adesnik, Hillel, Roger A. Nicoll, and Pamela M. England. 2005. “Photoinactivation of Native AMPA Receptors Reveals Their Real-Time Trafficking.” Neuron 48(6):977–85.
Equipment used: Spot illumination module (AiWon) - Andruchova, Olena, Marion Christine Höpflinger, Oleg Andruchov, and Stefan Galler. 2005. “No Effect of Twitchin Phosphorylation on the Rate of Myosin Head Detachment in Molluscan Catch Muscle: Are Myosin Heads Involved in the Catch State?” Pflügers Archiv : European Journal of Physiology 450(5):326–34.
Equipment used: Flash lamp system (JML-C2) - Diamond, Jeffrey S. 2005. “Deriving the Glutamate Clearance Time Course from Transporter Currents in CA1 Hippocampal Astrocytes: Transmitter Uptake Gets Faster during Development.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 25(11):2906–16.
Equipment used: Flash lamp system FlashMic (JML-C2) - Galler, Stefan, Marion Christine Höpflinger, Oleg Andruchov, Olena Andruchova, and Herbert Grassberger. 2005. “Effects of Vanadate, Phosphate and 2,3-Butanedione Monoxime (BDM) on Skinned Molluscan Catch Muscle.” Pflügers Archiv : European Journal of Physiology 449(4):372–83. Equipment used: Flash lamp system (JML-C2)
- Kang, Guoxin, Oleg G. Chepurny, Michael J. Rindler, Leon Collis, Zina Chepurny, Wen-Hong Li, Mark Harbeck, Michael W. Roe, and George G. Holz. 2005. “A CAMP and Ca2+ Coincidence Detector in Support of Ca2+-Induced Ca2+ Release in Mouse Pancreatic Beta Cells.” The Journal of Physiology 566(Pt 1):173–88.
Equipment used: Spot illumination module (AiWon) with Flash lamp system JML-C2 - Lou, Xuelin, Volker Scheuss, and Ralf Schneggenburger. 2005. “Allosteric Modulation of the Presynaptic Ca2+ Sensor for Vesicle Fusion.” Nature 435(7041):497–501.
Equipment used: Flash lamp system SP-20 - MacMillan, Debbi, Susan Currie, Karen N. Bradley, Thomas C. Muir, and John G. McCarron. 2005. “In Smooth Muscle, FK506-Binding Protein Modulates IP3 Receptor-Evoked Ca2+ Release by MTOR and Calcineurin.” Journal of Cell Science 118(Pt 23):5443–51.
Equipment used: Flash lamp system (JML-C2) - MacMillan, Debbi, Susan Chalmers, Thomas C. Muir, and John G. McCarron. 2005. “IP3-Mediated Ca2+ Increases Do Not Involve the Ryanodine Receptor, but Ryanodine Receptor Antagonists Reduce IP3-Mediated Ca2+ Increases in Guinea-Pig Colonic Smooth Muscle Cells.” The Journal of Physiology 569(Pt 2):533–44.
Equipment used: Flash lamp system (JML-C2) - Nache, Vasilica, Eckhard Schulz, Thomas Zimmer, Jana Kusch, Christoph Biskup, Rolf Koopmann, Volker Hagen, and Klaus Benndorf. 2005. “Activation of Olfactory-Type Cyclic Nucleotide-Gated Channels Is Highly Cooperative.” The Journal of Physiology 569(Pt 1):91–102. Equipment used: Flash lamp system JML-C2
- Ullrich, Nina D., Thomas Voets, Jean Prenen, Rudi Vennekens, Karel Talavera, Guy Droogmans, and Bernd Nilius. 2005. “Comparison of Functional Properties of the Ca2+-Activated Cation Channels TRPM4 and TRPM5 from Mice.” Cell Calcium 37(3):267–78.
Equipment used: Flash lamp system (JML-C2) - Wood, Christopher D., Takuya Nisihigaki, Toshiaki Furuta, Shoji A. Baba, and Alberto Darszon. 2005. “Real-Time Analysis of the Role of Ca 2 in Flagellar Movement and Motility in Single Sea Urchin Sperm.” Journal of Cell Biology 169(5):725–31. doi: 10.1083/jcb.200411001.
Equipment used: Flash lamp system (JML-C2)
2004
- Duguid, Ian C. and Trevor G. Smart. 2004. “Retrograde Activation of Presynaptic NMDA Receptors Enhances GABA Release at Cerebellar Interneuron-Purkinje Cell Synapses.” Nature Neuroscience 7(5):525–33.
Equipment used: Flash lamp system JML-C2 - Frère, Samuel G. A. and Anita Lüthi. 2004. “Pacemaker Channels in Mouse Thalamocortical Neurones Are Regulated by Distinct Pathways of CAMP Synthesis.” The Journal of Physiology 554(Pt 1):111–25.
Equipment used: Flash lamp system FlashMic (JML-C2) - Kurtz, Rafael. 2004. “Ca2+ Clearance in Visual Motion-Sensitive Neurons of the Fly Studied in Vivo by Sensory Stimulation and UV Photolysis of Caged Ca2+.” Journal of Neurophysiology 92(1):458–67. Equipment used: Spot illumination module (AiWon) with Flash lamp system JML-C2
- McCarron, John G., Debbi MacMillan, Karen N. Bradley, Susan Chalmers, and Thomas C. Muir. 2004. “Origin and Mechanisms of Ca2+ Waves in Smooth Muscle as Revealed by Localized Photolysis of Caged Inositol 1,4,5-Trisphosphate.” The Journal of Biological Chemistry 279(9):8417–27. Equipment used: Flash lamp system (JML-C2
- Nishigaki, Takuya, Christopher D. Wood, Yoshiro Tatsu, Noboru Yumoto, Toshiaki Furuta, David Elias, Kogiku Shiba, Shoji A. Baba, and Alberto Darszon. 2004. “A Sea Urchin Egg Jelly Peptide Induces a CGMP-Mediated Decrease in Sperm Intracellular Ca(2+) before Its Increase.” Developmental Biology 272(2):376–88.
Equipment used: Flash lamp system JML-C2 - Solzin, Johannes, Annika Helbig, Qui Van, Joel E. Brown, Eilo Hildebrand, Ingo Weyand, and U. Benjamin Kaupp. 2004. “Revisiting the Role of H+ in Chemotactic Signaling of Sperm.” The Journal of General Physiology 124(2):115–24.
Equipment used: Flash lamp system JML-C2 - Thoreson, Wallace B., Katalin Rabl, Ellen Townes-Anderson, and Ruth Heidelberger. 2004. “A Highly Ca2+-Sensitive Pool of Vesicles Contributes to Linearity at the Rod Photoreceptor Ribbon Synapse.” Neuron 42(4):595–605.
Equipment used: Flash lamp system (JML-C2) - Wan, Qun-Fang, Yongming Dong, Hua Yang, Xuelin Lou, Jiuping Ding, and Tao Xu. 2004. “Protein Kinase Activation Increases Insulin Secretion by Sensitizing the Secretory Machinery to Ca2+.” The Journal of General Physiology 124(6):653–62.
Equipment used: Flash lamp system (JML-C2) - Zheng, Ji-Jian, Seunghoon Lee, and Z. Jimmy Zhou. 2004. “A Developmental Switch in the Excitability and Function of the Starburst Network in the Mammalian Retina.” Neuron 44(5):851–64. Equipment used: Spot illumination module (AiWon) with Flash lamp system JML-C2
2003
- Felmy, Felix, Erwin Neher, and Ralf Schneggenburger. 2003. “Probing the Intracellular Calcium Sensitivity of Transmitter Release during Synaptic Facilitation.” Neuron 37(5):801–11.
Equipment used: Flash lamp system SP-20 - Sørensen, Jakob B., Rafael Fernández-Chacón, Thomas C. Südhof, and Erwin Neher. 2003. “Examining Synaptotagmin 1 Function in Dense Core Vesicle Exocytosis under Direct Control of Ca2+.” The Journal of General Physiology 122(3):265–76.
Equipment used: Flash lamp system (JML-C2) with light guide adapter LGA - Wölfel, Markus and Ralf Schneggenburger. 2003. “Presynaptic Capacitance Measurements and Ca2+ Uncaging Reveal Submillisecond Exocytosis Kinetics and Characterize the Ca2+ Sensitivity of Vesicle Pool Depletion at a Fast CNS Synapse.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 23(18):7059–68.
Equipment used: Flash lamp system SP-20
2002
- Bradley, Karen N., Elaine R. M. Flynn, Thomas C. Muir, and John G. McCarron. 2002. “Ca(2+) Regulation in Guinea-Pig Colonic Smooth Muscle: The Role of the Na(+)-Ca(2+) Exchanger and the Sarcoplasmic Reticulum.” The Journal of Physiology 538(Pt 2):465–82.
Equipment used: Flash lamp system (JML-C2) - McCarron, John G., John W. Craig, Karen N. Bradley, and Thomas C. Muir. 2002. “Agonist-Induced Phasic and Tonic Responses in Smooth Muscle Are Mediated by InsP(3).” Journal of Cell Science 115(Pt 10):2207–18.
Equipment used: Flash lamp system (JML-C2) - Ng, Yuen-Keng, Xinghua Lu, Simon C. Watkins, Graham C. R. Ellis-Davies, and Edwin S. Levitan. 2002. “Nerve Growth Factor-Induced Differentiation Changes the Cellular Organization of Regulated Peptide Release by PC12 Cells.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 22(10):3890–97.
Equipment used: Flash lamp system (JML-C2) - Tatsu, Yoshiro, Takuya Nishigaki, Alberto Darszon, and Noboru Yumoto. 2002. “A Caged Sperm-Activating Peptide That Has a Photocleavable Protecting Group on the Backbone Amide.” FEBS Letters 525(1–3):20–24.
Equipment used: Flash lamp system JML-C2 - Xu, Jianhua, Yimei Xu, Graham C. R. Ellis-Davies, George J. Augustine, and Frederick W. Tse. 2002. “Differential Regulation of Exocytosis by Alpha- and Beta-SNAPs.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 22(1):53–61.
Equipment used: Flash lamp system XF-10 (JML-C2)
2001
- Barg, S., X. Ma, L. Eliasson, J. Galvanovskis, S. O. Göpel, S. Obermüller, J. Platzer, Erik Renström, M. Trus, D. Atlas, J. Striessnig, and Patrik Rorsman. 2001. “Fast Exocytosis with Few Ca(2+) Channels in Insulin-Secreting Mouse Pancreatic B Cells.” Biophysical Journal 81(6):3308–23. Equipment used: Flash lamp system XF-10 (JML-C2)
- Beutner, Dirk, Thomas Voets, Erwin Neher, and Tobias Moser. 2001. “Calcium Dependence of Exocytosis and Endocytosis at the Cochlear Inner Hair Cell Afferent Synapse.” Neuron 29(3):681–90.
Equipment used: Flash lamp system (JML-C2) with light guide adapter LGA - Chen, P., T. C. Hwang, and K. D. Gillis. 2001. “The Relationship between CAMP, Ca(2)+, and Transport of CFTR to the Plasma Membrane.” The Journal of General Physiology 118(2):135–44. Equipment used: Flash lamp system JML-C1 (JML-C2)
- Flynn, Elaine R. M., Karen N. Bradley, Thomas C. Muir, and John G. McCarron. 2001. “Functionally Separate Intracellular Ca2+ Stores in Smooth Muscle.” The Journal of Biological Chemistry 276(39):36411–18.
Equipment used: Flash lamp system (JML-C2) - Hoskins, B. K., C. C. Ashley, Gert Rapp, and P. J. Griffiths. 2001. “Time-Resolved X-Ray Diffraction by Skinned Skeletal Muscle Fibers during Activation and Shortening.” Biophysical Journal 80(1):398–414.
Equipment used: Flash lamp system (JML-C2) - Lagostena, Laura, J. F. Ashmore, B. Kachar, and F. Mammano. 2001. “Purinergic Control of Intercellular Communication between Hensen’s Cells of the Guinea-Pig Cochlea.” The Journal of Physiology 531(Pt 3):693–706.
Equipment used: Spot illumination module (AiWon) with flash lamp system JML-C2 - Lancaster, B., H. Hu, G. M. Ramakers, and J. F. Storm. 2001. “Interaction between Synaptic Excitation and Slow Afterhyperpolarization Current in Rat Hippocampal Pyramidal Cells.” The Journal of Physiology 536(Pt 3):809–23.
Equipment used: Flash lamp system XF-10 (JML-C2) - Kishimoto, T., T. T. Liu, Y. Ninomiya, H. Takagi, T. Yoshioka, Graham C. R. Ellis-Davies, Y. Miyashita, and H. Kasai. 2001. “Ion Selectivities of the Ca(2+) Sensors for Exocytosis in Rat Phaeochromocytoma Cells.” The Journal of Physiology 533(Pt 3):627–37.
Equipment used: Flash lamp system XF-10 (JML-C2)
1998
- Rapp, Gert. 1998. “Flash Lamp-Based Irradiation of Caged Compounds.” Methods in Enzymology 291:202–22.
Equipment used: Flash lamp systems
1994
- Heinemann, C., Robert H. Chow, Erwin Neher, and R. S. Zucker. 1994. “Kinetics of the Secretory Response in Bovine Chromaffin Cells Following Flash Photolysis of Caged Ca2+.” Biophysical Journal 67(6):2546–57.
Equipment used: Flash lamp system (JML-C2) with light guide adapter LGA
1993
- Callaway, E. M. and L. C. Katz. 1993. “Photostimulation Using Caged Glutamate Reveals Functional Circuitry in Living Brain Slices.” Proceedings of the National Academy of Sciences of the United States of America 90(16):7661–65.
Equipment used: Flash lamp system (JML-C2)
1991
- Ménétret, J. F., W. Hofmann, R. R. Schröder, G. Rapp, and R. S. Goody. 1991. “Time-Resolved Cryo-Electron Microscopic Study of the Dissociation of Actomyosin Induced by Photolysis of Photolabile Nucleotides.” Journal of Molecular Biology 219(2):139–44.
Equipment used: Flash lamp system (JML-C2)
Optogenetics & Photostimulation
and photoconversion & -switching application here
2024
- Bertho, Maëlle, Vanessa Caldeira, Li-Ju Hsu, Peter Löw, Lotta Borgius, and Ole Kiehn. “Excitatory Spinal Lhx9-Derived Interneurons Modulate Locomotor Frequency in Mice.” The Journal of Neuroscience (September):e1607232024. doi: 10.1523/JNEUROSCI.1607-23.2024.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Harb, Ali, Charlotte Tacke, Behnam Vafadari, and Swen Hülsmann. “The Relative Contribution of Glycine–GABA Cotransmission in the Core of the Respiratory Network.” International Journal of Molecular Sciences 25(6):3128. doi: 10.3390/ijms25063128.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-488. - Wang, Jiali, Eric Platz‐Baudin, Erik Noetzel, Andreas Offenhäusser, and Vanessa Maybeck. “Expressing Optogenetic Actuators Fused to N‐terminal Mucin Motifs Delivers Targets to Specific Subcellular Compartments in Polarized Cells.” Advanced Biology 8(3). doi: 10.1002/adbi.202300428.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2023
- Hsu, Li-Ju, Maëlle Bertho, and Ole Kiehn. 2023 “Deconstructing the Modular Organization and Real-Time Dynamics of Mammalian Spinal Locomotor Networks.” Nature Communications 14(1):873. doi: 10.1038/s41467-023-36587-w.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Kim, Seung Chan, Scott J. Mitchell, Seema Qamar, Daniel J. Whitcomb, Marc-David Ruepp, Peter St George-Hyslop, and Kwangwook Cho. 2023 “Mimicking Hypomethylation of FUS Requires Liquid–Liquid Phase Separation to Induce Synaptic Dysfunctions.” Acta Neuropathologica Communications 11(1):199. doi: 10.1186/s40478-023-01703-w.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-647 - Rook, Matthew L., Tyler W. McCullock, Tyler Couch, John D. Lueck, and David M. MacLean. 2023. “Photomodulation of the ASIC1a Acidic Pocket Destabilizes the Open State.” Protein Science 32(11). doi: 10.1002/pro.4800.
Equipment used: Illumination system KSL-2 with UV light 365nm (KSL-2) - Traunmüller, Lisa, Jan Schulz, Raul Ortiz, Huijuan Feng, Elisabetta Furlanis, Andrea M. Gomez, Dietmar Schreiner, Josef Bischofberger, Chaolin Zhang, and Peter Scheiffele. 2023 “A Cell-Type-Specific Alternative Splicing Regulator Shapes Synapse Properties in a Trans-Synaptic Manner.” Cell Reports 42(3):112173. doi: 10.1016/j.celrep.2023.112173.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Wang, Jiali, Eric Platz‐Baudin, Erik Noetzel, Andreas Offenhäusser, and Vanessa Maybeck. 2023. “Expressing Optogenetic Actuators Fused to N‐terminal Mucin Motifs Delivers Targets to Specific Subcellular Compartments in Polarized Cells.” Advanced Biology. doi: 10.1002/adbi.202300428.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2022
- Hauser, David, Katharina Behr, Kohtarou Konno, Dietmar Schreiner, Alexander Schmidt, Masahiko Watanabe, Josef Bischofberger, and Peter Scheiffele. “Targeted Proteoform Mapping Uncovers Specific Neurexin-3 Variants Required for Dendritic Inhibition.” Neuron 110(13):2094-2109.e10. doi: 10.1016/j.neuron.2022.04.017.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Islam, Md Tarikul, Florian Rumpf, Yusuke Tsuno, Shota Kodani, Takeshi Sakurai, Ayako Matsui, Takashi Maejima, and Michihiro Mieda. 2022 “Vasopressin Neurons in the Paraventricular Hypothalamus Promote Wakefulness via Lateral Hypothalamic Orexin Neurons.” Current Biology. doi: 10.1016/j.cub.2022.07.020.
Equipment used: diode single line laser DL-473 - Islam, Md Tarikul, Takashi Maejima, Ayako Matsui, and Michihiro Mieda. 2022 “Paraventricular Hypothalamic Vasopressin Neurons Induce Self-Grooming in Mice.” Molecular Brain 15(1):1–14. doi: 10.1186/s13041-022-00932-9.
Equipment used: diode single line laser DL-473 - Shipton, Olivia A., Clara S. Tang, Ole Paulsen, and Mariana Vargas-Caballero. 2022 “Differential Vulnerability of Hippocampal CA3-CA1 Synapses to Aβ.” Acta Neuropathologica Communications 10(1):45. doi: 10.1186/s40478-022-01350-7.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Tjiang, Noah, and Hans Zempel. 2022. “A Mitochondria Cluster at the Proximal Axon Initial Segment Controls Axodendritic TAU Trafficking in Rodent Primary and Human IPSC-Derived Neurons.” Cellular and Molecular Life Sciences 79(2):120. doi: 10.1007/s00018-022-04150-3.
Equipment used: Photomanipulation system UGA-40 Caliburn (UGA-42 Firefly) with pulsed UV laser DPSL-355 (UGA-42 Caliburn) - Wang, Shan, Jon Ruben van Rhijn, Ibrahim Akkouh, Naoki Kogo, Nadine Maas, Anna Bleeck, Irene Santisteban Ortiz, Elly Lewerissa, Ka Man Wu, Chantal Schoenmaker, Srdjan Djurovic, Hans van Bokhoven, Tjitske Kleefstra, Nael Nadif Kasri, and Dirk Schubert. “Loss-of-Function Variants in the Schizophrenia Risk Gene SETD1A Alter Neuronal Network Activity in Human Neurons through the CAMP/PKA Pathway.” Cell Reports 39(5):110790. doi: 10.1016/j.celrep.2022.110790.
Equipment used: Illumination system KSL-2 with 470nm (KSL-2)
2021
- Aksoy-Aksel, Ayla, Andrea Gall, Anna Seewald, Francesco Ferraguti, and Ingrid Ehrlich. 2021 “Midbrain Dopaminergic Inputs Gate Amygdala Intercalated Cell Clusters by Distinct and Cooperative Mechanisms in Male Mice.” ELife 10:1–28. doi: 10.7554/eLife.63708.
Equipment used: Illumination system KSL-2 with 470nm (KSL-2) - Anni, Daniela, Eva-Maria Weiss, Debarpan Guhathakurta, Yagiz Enes Akdas, Julia Klueva, Stefanie Zeitler, Maria Andres-Alonso, Tobias Huth, and Anna Fejtova. 2021. “Aβ1-16 Controls Synaptic Vesicle Pools at Excitatory Synapses via Cholinergic Modulation of Synapsin Phosphorylation.” Cellular and Molecular Life Sciences (0123456789). doi: 10.1007/s00018-021-03835-5.
Equipment used: high power short wave UV illumination system UVICO (UVICO-2) - Hay, Y. Audrey, Nicolas Deperrois, Tanja Fuchsberger, Thomas Matthew Quarrell, Anna-Lucia Koerling, and Ole Paulsen. 2021 “Thalamus Mediates Neocortical Down State Transition via GABAB-Receptor-Targeting Interneurons.” Neuron 109(17):2682-2690.e5. doi: 10.1016/j.neuron.2021.06.030.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Kim, Hyejeong, Hyunah Kwon, Ryungeun Song, Seonghun Shin, So-Young Ham, Hee-Deung Park, Jinkee Lee, Peer Fischer, and Eberhard Bodenschatz. “Hierarchical Optofluidic Microreactor for Water Purification Using an Array of TiO2 Nanostructures.” Npj Clean Water 5(1):62. doi: 10.1038/s41545-022-00204-y.
Equipment used: high power short wave UV illumination system UVICO (UVICO-2) - Li, Yan, Pei-ju Chen, Tzu-yang Lin, Chun-Yuan Ting, Pushpanathan Muthuirulan, Randall Pursley, Marko Ilić, Primož Pirih, Michael S. Drews, Kaushiki P. Menon, Kai G. Zinn, Thomas Pohida, Alexander Borst, and Chi-hon Lee. 2021. “Neural Mechanism of Spatio-Chromatic Opponency in the Drosophila Amacrine Neurons.” Current Biology 1–13. doi: 10.1016/j.cub.2021.04.068.
Equipment used: Shape Illumination Photomanipulation system UGA-42 Geo with diode single line laser DL-594 - Lodge, Meredith, Maria-Clemencia Hernandez, Jan M. Schulz, and Josef Bischofberger. 2021. “Sparsification of AP Firing in Adult-Born Hippocampal Granule Cells via Voltage-Dependent Α5-GABAA Receptors.” Cell Reports 37(1):109768. doi: 10.1016/j.celrep.2021.109768.
Equipment used: Spot illumination module(AiWon) with diode single line laser DL-473 - Ohara, Shinya, Stefan Blankvoort, Rajeevkumar R. Nair, Maximiliano J. Nigro, Eirik S. Nilssen, Clifford Kentros, and Menno P. Witter. 2021. “Local Projections of Layer Vb-to-Va Are More Prominent in Lateral than in Medial Entorhinal Cortex.” ELife 10. https://doi.org/10.7554/eLife.67262.
Equipment used: Shape Illumination Photomanipulation system UGA-42 Geo with diode single line laser DL-473 - Pirger Z, László Z, Naskar S, Crossley M, O’Shea M, Benjamin PR, Kemenes G, Kemenes I (2021) Interneuronal mechanisms for learning-induced switch in a sensory response that anticipates changes in behavioral outcomes. Curr Biol:1–8 Available at: https://linkinghub.elsevier.com/retrieve/pii/S0960982221001378.
Equipment used: Flash lamp system (JML-C2) - Schulz, Jan M., Jim W. Kay, Josef Bischofberger, and Matthew E. Larkum. 2021. “GABAB Receptor-Mediated Regulation of Dendro-Somatic Synergy in Layer 5 Pyramidal Neurons.” Frontiers in Cellular Neuroscience 15(August):1–19. doi: 10.3389/fncel.2021.718413.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Yu, Szi-chieh, Jana F. Liewald, Jiajie Shao, Wagner Steuer Costa, and Alexander Gottschalk. 2021. “Synapsin Is Required for Dense Core Vesicle Capture and CAMP-Dependent Neuropeptide Release.” The Journal of Neuroscience JN-RM-2631-20. doi: 10.1523/JNEUROSCI.2631-20.2021.
Equipment used: Illumination system KSL-2 with 470nm (KSL-2)
2020
- Apóstolo, Nuno, Samuel N. Smukowski, Jeroen Vanderlinden, Giuseppe Condomitti, Vasily Rybakin, Jolijn ten Bos, Laura Trobiani, Sybren Portegies, Kristel M. Vennekens, Natalia V. Gounko, Davide Comoletti, Keimpe D. Wierda, Jeffrey N. Savas, and Joris de Wit. 2020 “Synapse Type-Specific Proteomic Dissection Identifies IgSF8 as a Hippocampal CA3 Microcircuit Organizer.” Nature Communications 11(1).
Equipment used: Photomanipulation system UGA-42 Geo with diode single line laser DL-473. - Belyy, Vladislav, Ngoc Han Tran, and Peter Walter. 2020. “Quantitative Microscopy Reveals Dynamics and Fate of Clustered IRE1α.” Proceedings of the National Academy of Sciences of the United States of America 117(3):1533–42.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-405 - Bocchero, Ulisse, Fabio Falleroni, Simone Mortal, Yunzhen Li, Dan Cojoc, Trevor Lamb, and Vincent Torre. 2020. “Mechanosensitivity Is an Essential Component of Phototransduction in Vertebrate Rods” edited by S. Hattar. PLOS Biology 18(7):e3000750. doi: 10.1371/journal.pbio.3000750.
Equipment used: 470nm LED for diffuse light stimulation - Braganza, Oliver, Daniel Mueller-Komorowska, Tony Kelly, and Heinz Beck. 2020. “Quantitative Properties of a Feedback Circuit Predict Frequency-Dependent Pattern Separation.” ELife 9.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Chen, Lili, Ya Wang, and Shangbang Gao. 2020. “Minimum Number of Synaptic Vesicles for the Initiation of a Single Action Potential at C. Elegans Neuromuscular Junction.” MicroPublication Biology (C):8–10.
Equipment used: Illumination system KSL-2 with 470nm (KSL-2) - Cregg, Jared M., Roberto Leiras, Alexia Montalant, Paulina Wanken, Ian R. Wickersham, and Ole Kiehn. 2020. “Brainstem Neurons That Command Mammalian Locomotor Asymmetries.” Nature Neuroscience.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Geschwill, Pascal, Martin E. Kaiser, Paul Grube, Nadja Lehmann, Christian Thome, Andreas Draguhn, Jan Oliver Hollnagel, and Martin Both. 2020. “Synchronicity of Excitatory Inputs Drives Hippocampal Networks to Distinct Oscillatory Patterns.” Hippocampus 30(10):1044–57.
Equipment used: diode single line laser DL-473 - Imig, Cordelia, Francisco José López-Murcia, Lydia Maus, Inés Hojas García-Plaza, Lena Sünke Mortensen, Manuela Schwark, Valentin Schwarze, Julie Angibaud, U. Valentin Nägerl, Holger Taschenberger, Nils Brose, and Benjamin H. Cooper. 2020. “Ultrastructural Imaging of Activity-Dependent Synaptic Membrane-Trafficking Events in Cultured Brain Slices.” Neuron 1–18.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) - Jullié, Damien, Miriam Stoeber, Jean Baptiste Sibarita, Hanna L. Zieger, Thomas M. Bartol, Seksiri Arttamangkul, Terrence J. Sejnowski, Eric Hosy, and Mark von Zastrow. 2020. “A Discrete Presynaptic Vesicle Cycle for Neuromodulator Receptors.” Neuron 105(4):663-677.e8.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Kim, Hyejeong, Seong-min Jo, Fanlong Meng, Yinzhou Guo, Héloïse Thérien-Aubin, Ramin Golestanian, Katharina Landfester, and Eberhard Bodenschatz. 2020. “One-Step Generation of Core-Gap-Shell Microcapsules for Stimuli-Responsive Biomolecular Sensing.” Advanced Functional Materials 2006019:2006019.
Equipment used: high power short wave UV illumination system UVICO (UVICO-2) - Pfisterer, Karin, James Levitt, Campbell D. Lawson, Richard J. Marsh, John M. Heddleston, Eric Wait, Simon Morris Ameer-Beg, Susan Cox, and Maddy Parsons. 2020. “FMNL2 Regulates Dynamics of Fascin in Filopodia.” The Journal of Cell Biology 219(5).
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-405 - Rook, Matthew L., Abby Williamson, John D. Lueck, Maria Musgaard, and David M. Maclean. 2020. “Β11-12 Linker Isomerization Governs Acid-Sensing Ion Channel Desensitization and Recovery.” ELife 9:1–24.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) - Sun, Yu-Yo, Yi-Min Kuo, Hong-Ru Chen, Jonah C. Short-Miller, Marchelle R. Smucker, and Chia-Yi Kuan. 2020. “A Murine Photothrombotic Stroke Model with an Increased Fibrin Content and Improved Responses to TPA-Lytic Treatment.” Blood Advances 4(7):1222–31. doi: 10.1182/bloodadvances.2019000782.
Equipment used: Spot illumination module(AiWon) with diode single line laser DL-561 and Sutter BOB microscope - Tudeau, Laetitia, Mario A. Acuña, Gioele W. Albisetti, Elena Neumann, William T. Ralvenius, Louis Scheurer, Michael Poe, James M. Cook, Helge C. Johannssen, and Hanns Ulrich Zeilhofer. 2020. “Mice Lacking Spinal Α2GABAA Receptors: Altered GABAergic Neurotransmission, Diminished GABAergic Antihyperalgesia, and Potential Compensatory Mechanisms Preventing a Hyperalgesic Phenotype.” Brain Research 1741(April):146889.
Equipment used: Shape Illumination Photomanipulation system UGA-42 Geo with diode single line laser DL-473
2019
- Bocchero, Ulisse, Beatrice M. Tam, Colette N. Chiu, Vincent Torre, and Orson L. Moritz. 2019. “Electrophysiological Changes during Early Steps of Retinitis Pigmentosa.” Investigative Ophthalmology and Visual Science 60(4):933–43.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) - Doan, Thanh P., Maria J. Lagartos-Donate, Eirik S. Nilssen, Shinya Ohara, and Menno P. Witter. 2019. “Convergent Projections from Perirhinal and Postrhinal Cortices Suggest a Multisensory Nature of Lateral, but Not Medial, Entorhinal Cortex.” Cell Reports 29(3):617-627.e7.
Equipment used: Shape Illumination Photomanipulation system UGA-42 Geo with diode single line laser DL-473 - Dvorzhak, Anton, Nordine Helassa, Katalin Török, Dietmar Schmitz, and Rosemarie Grantyn. 2019. “Single Synapse Indicators of Impaired Glutamate Clearance Derived from Fast IGluu Imaging of Cortical Afferents in the Striatum of Normal and Huntington (Q175) Mice.” Journal of Neuroscience 39(20):3970–82.
Equipment used: Photomanipulation system UGA-42 Firefly - Grandjean, Joanes, Alberto Corcoba, Martin C. Kahn, A. Louise Upton, Evan S. Deneris, Erich Seifritz, Fritjof Helmchen, Edward O. Mann, Markus Rudin, and Bechara J. Saab. 2019. “A Brain-Wide Functional Map of the Serotonergic Responses to Acute Stress and Fluoxetine.” Nature Communications 10(1):350.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Kodani, Shota, Shingo Soya, and Takeshi Sakurai. 2019. “Optogenetic Manipulation of Neural Circuits during Monitoring Sleep/ Wakefulness States in Mice.” Journal of Visualized Experiments 2019(148):1–2.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Stahlberg, Markus A., Charu Ramakrishnan, Katrin I. Willig, Edward S. Boyden, Karl Deisseroth, and Camin Dean. 2019. “Investigating the Feasibility of Channelrhodopsin Variants for Nanoscale Optogenetics.” Neurophotonics 6(01):1.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-594 - Steuer Costa, Wagner, Petrus Van der Auwera, Caspar Glock, Jana F. Liewald, Maximilian Bach, Christina Schüler, Sebastian Wabnig, Alexandra Oranth, Florentin Masurat, Henrik Bringmann, Liliane Schoofs, Ernst H. K. Stelzer, Sabine C. Fischer, and Alexander Gottschalk. 2019. “A GABAergic and Peptidergic Sleep Neuron as a Locomotion Stop Neuron with Compartmentalized Ca2+ Dynamics.” Nature Communications 10(1).
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2)
2018
- Bergs, Amelie, Christian Schultheis, Elisabeth Fischer, Satoshi P. Tsunoda, Karen Erbguth, Steven J. Husson, Elena Govorunova, John L. Spudich, Georg Nagel, Alexander Gottschalk, and Jana F. Liewald. 2018. “Rhodopsin Optogenetic Toolbox v2.0 for Light-Sensitive Excitation and Inhibition in Caenorhabditis Elegans.” PLoS ONE 13(2):1–24.
Equipment used: Single wavelength LED Illumination system KSL-2 (KSL-2) - Keplinger, Stefan, Barbara Beiderbeck, Stylianos Michalakis, Martin Biel, Benedikt Grothe, and Lars Kunz. 2018. “Optogenetic Control of Neural Circuits in the Mongolian Gerbil.” Frontiers in Cellular Neuroscience 12(April):1–17.
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) - Kotzadimitriou, Dimitrios, Wiebke Nissen, Melinda Paizs, Kathryn Newton, Paul J. Harrison, Ole Paulsen, and Karri Lamsa. “Neuregulin 1 Type i Overexpression Is Associated with Reduced NMDA Receptor–Mediated Synaptic Signaling in Hippocampal Interneurons Expressing PV or CCK.” ENeuro 5(2).
Equipment used: Spot illumination module (AiWon) with diode single line laser - Lim, Lynette, Janelle M. P. Pakan, Martijn M. Selten, André Marques-Smith, Alfredo Llorca, Sung Eun Bae, Nathalie L. Rochefort, and Oscar Marín. 2018. “Optimization of Interneuron Function by Direct Coupling of Cell Migration and Axonal Targeting.” Nature Neuroscience 21(7):920–31.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Moretti, Federico Andrea, Sarah Klapproth, Raphael Ruppert, Andreas Margraf, Jasmin Weber, Robert Pick, Christoph Scheiermann, Markus Sperandio, Reinhard Fässler, and Markus Moser. “Differential Requirement of Kindlin-3 for T Cell Progenitor Homing to the Non-Vascularized and Vascularized Thymus.” ELife 7:1–28.
Equipment used: Flash lamp system (JML-C2) - Nilssen, Eirik S., Bente Jacobsen, Gunhild Fjeld, Rajeevkumar R. Nair, Stefan Blankvoort, Clifford Kentros, and Menno P. Witter. 2018. “Inhibitory Connectivity Dominates the Fan Cell Network in Layer Ii of Lateral Entorhinal Cortex.” Journal of Neuroscience 38(45):9712–27.
Equipment used: Shape Illumination Photomanipulation system UGA-42 Geo with diode single line laser DL-473 - Saito, Yuki C., Takashi Maejima, Mitsuhiro Nishitani, Emi Hasegawa, Yuchio Yanagawa, Michihiro Mieda, and Takeshi Sakurai. 2018. “Monoamines Inhibit GABAergic Neurons in Ventrolateral Preoptic Area That Make Direct Synaptic Connections to Hypothalamic Arousal Neurons.” Journal of Neuroscience 38(28):6366–78.
Equipment used: Single wavelength LED Illumination system KSL-2 (KSL-2) - Scheyltjens, Isabelle, Samme Vreysen, Chris Van den Haute, Victor Sabanov, Detlef Balschun, Veerle Baekelandt, and Lutgarde Arckens. 2018. “Transient and Localized Optogenetic Activation of Somatostatin-Interneurons in Mouse Visual Cortex Abolishes Long-Term Cortical Plasticity Due to Vision Loss.” Brain Structure and Function 223(5):2073–95.
Equipment used: Single wavelength LED Illumination system KSL-2 (KSL-2) - Schulz, Jan M., Frederic Knoflach, Maria Clemencia Hernandez, and Josef Bischofberger. 2018. “Dendrite-Targeting Interneurons Control Synaptic NMDA-Receptor Activation via Nonlinear Α5-GABAA Receptors.” Nature Communications 9(1).
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Yu, Szi Chieh, Barbara Jánosi, Jana F. Liewald, Sebastian Wabnig, and Alexander Gottschalk. 2018. “Endophilin A and B Join Forces with Clathrin to Mediate Synaptic Vesicle Recycling in Caenorhabditis Elegans.” Frontiers in Molecular Neuroscience 11.
Equipment used: Single wavelength LED Illumination system KSL-2 (KSL-2) - Zhang, Yan Feng, John N. J. Reynolds, and Stephanie J. Cragg. 2018. “Pauses in Cholinergic Interneuron Activity Are Driven by Excitatory Input and Delayed Rectification, with Dopamine Modulation.” Neuron 98(5):918-925.e3.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473
2017
- Bellardita, Carmelo, Vittorio Caggiano, Roberto Leiras, Vanessa Caldeira, Andrea Fuchs, Julien Bouvier, Peter Löw, and Ole Kiehn. 2017. “Spatiotemporal Correlation of Spinal Network Dynamics Underlying Spasms in Chronic Spinalized Mice.” ELife 6:1–23.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 and DL-550 - Fischer, Elisabeth, Alexander Gottschalk, and Christina Schüler. “An Optogenetic Arrhythmia Model to Study Catecholaminergic Polymorphic Ventricular Tachycardia Mutations.” Scientific Reports 7(1):1–12.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) - Guy, Julien, Alexandra Sachkova, Martin Möck, Mirko Witte, Robin J. Wagener, and Jochen F. Staiger. 2017. “Intracortical Network Effects Preserve Thalamocortical Input Efficacy in a Cortex Without Layers.” Cerebral Cortex 27(10):4851–66.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Hasegawa, Emi, Takashi Maejima, Takayuki Yoshida, Olivia A. Masseck, Stefan Herlitze, Mitsuhiro Yoshioka, Takeshi Sakurai, and Michihiro Mieda. 2017. “Serotonin Neurons in the Dorsal Raphe Mediate the Anticataplectic Action of Orexin Neurons by Reducing Amygdala Activity.” Proceedings of the National Academy of Sciences of the United States of America 114(17):E3526–35. doi: 10.1073/pnas.1614552114.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Jin, Lei, Wienke Lange, Annika Kempmann, Vanessa Maybeck, Anne Günther, Nadine Gruteser, Arnd Baumann, and Andreas Offenhäusser. 2016. “High-Efficiency Transduction and Specific Expression of ChR2opt for Optogenetic Manipulation of Primary Cortical Neurons Mediated by Recombinant Adeno-Associated Viruses.” Journal of Biotechnology 233:171–80.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Miller, Oliver H., Andreas Bruns, Imen Ben Ammar, Thomas Mueggler, and Benjamin J. Hall. “Synaptic Regulation of a Thalamocortical Circuit Controls Depression-Related Behavior.” Cell Reports 20(8):1867–80.
Equipment used: Shape Illumination Photomanipulation system UGA-42 Geo with diode single line laser DL-473 - Westhoff, Maartje, Christopher I. Murray, Jodene Eldstrom, and David Fedida. 2017. “Photo-Cross-Linking of IKs Demonstrates State-Dependent Interactions between KCNE1 and KCNQ1.” Biophysical Journal 113(2):415–25.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2)
2016
- Anastasiades, Paul G., Andre Marques-Smith, Daniel Lyngholm, Tom Lickiss, Sayda Raffiq, Dennis Katzel, Gero Miesenbock, and Simon J. B. Butt. 2016. “GABAergic Interneurons Form Transient Layer-Specific Circuits in Early Postnatal Neocortex.” Nature Communications 7(1):10584.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with pulsed 30mW UV laser DPSS-355/30 - Gökçe, Onur, Tobias Bonhoeffer, and Volker Scheuss. 2016. “Clusters of Synaptic Inputs on Dendrites of Layer 5 Pyramidal Cells in Mouse Visual Cortex.” ELife 5(JULY):1–14.
Equipment used: Single wavelength LED Illumination system KSL-2 (KSL-2) - Jin, Lei, Wienke Lange, Annika Kempmann, Vanessa Maybeck, Anne Günther, Nadine Gruteser, Arnd Baumann, and Andreas Offenhäusser. 2016. “High-Efficiency Transduction and Specific Expression of ChR2opt for Optogenetic Manipulation of Primary Cortical Neurons Mediated by Recombinant Adeno-Associated Viruses.” Journal of Biotechnology 233:171–80.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Kosillo, Polina, Yan Feng Zhang, Sarah Threlfell, and Stephanie J. Cragg. 2016. “Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons.” Cerebral Cortex 26(11):4160–69.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Maybeck, V., J. Schnitker, W. Li, M. Heuschkel, and A. Offenhausser. 2016. “An Evaluation of Extracellular MEA versus Optogenetic Stimulation of Cortical Neurons.” Biomedical Physics and Engineering Express.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Pabst, Milan, Oliver Braganza, Holger Dannenberg, Wen Hu, Leonie Pothmann, Jurij Rosen, Istvan Mody, Karen van Loo, Karl Deisseroth, Albert J. Becker, Susanne Schoch, and Heinz Beck. 2016. “Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus.” Neuron 90(4):853–65.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Roberts, William M., Steven B. Augustine, Kristy J. Lawton, Theodore H. Lindsay, Tod R. Thiele, Eduardo J. Izquierdo, Serge Faumont, Rebecca A. Lindsay, Matthew Cale Britton, Navin Pokala, Cornelia I. Bargmann, and Shawn R. Lockery. 2016. “A Stochastic Neuronal Model Predicts Random Search Behaviors at Multiple Spatial Scales in C. Elegans.” ELife 5(JANUARY2016):1–41.
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) - Walker, F., M. Möck, M. Feyerabend, J. Guy, R. J. Wagener, D. Schubert, J. F. Staiger, and M. Witte. 2016. “Parvalbumin-and Vasoactive Intestinal Polypeptide-Expressing Neocortical Interneurons Impose Differential Inhibition on Martinotti Cells.” Nature Communications 7.(1):13664.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-405 and DL-473
2015
- Gao, Shangbang, Lin Xie, Taizo Kawano, Michelle D. Po, Sihui Guan, and Mei Zhen. 2015. “The NCA Sodium Leak Channel Is Required for Persistent Motor Circuit Activity That Sustains Locomotion.” Nature Communications 6(May).
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) - Kosillo, Polina, Katherine R. Brimblecombe, Sarah Threlfell, and Stephanie J. Cragg. 2015. “Coupling voltammetry with optogenetics to reveal axonal control of dopamine transmission by striatal acetylcholine.” in Compendium of In Vivo Monitoring in Real-Time Molecular Neuroscience by Wilson, George S Michael, Adrian C.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473
2014
- Azimihashemi, N., K. Erbguth, A. Vogt, T. Riemensperger, E. Rauch, D. Woodmansee, J. Nagpal, M. Brauner, M. Sheves, A. Fiala, L. Kattner, D. Trauner, P. Hegemann, A. Gottschalk, and J. F. Liewald. 2014. “Synthetic Retinal Analogues Modify the Spectral and Kinetic Characteristics of Microbial Rhodopsin Optogenetic Tools.” Nature Communications 5.
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) - Drews, A., F. Mohr, O. Rizun, T. F. J. Wagner, S. Dembla, S. Rudolph, S. Lambert, M. Konrad, S. E. Philipp, M. Behrendt, S. Marchais-Oberwinkler, D. F. Covey, and J. Oberwinkler. 2014. “Structural Requirements of Steroidal Agonists of Transient Receptor Potential Melastatin 3 (TRPM3) Cation Channels.” British Journal of Pharmacology 171(4):1019–32.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Figueiredo, Melina, Samantha Lane, Randy F. Stout, Beihui Liu, Vladimir Parpura, Anja G. Teschemacher, and Sergey Kasparov. 2014. “Comparative Analysis of Optogenetic Actuators in Cultured Astrocytes.” Cell Calcium 56(3):208–14.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Klippenstein, Viktoria, Valentina Ghisi, Marcus Wietstruk, and Andrew J. R. Plested. 2014. “Photoinactivation of Glutamate Receptors by Genetically Encoded Unnatural Amino Acids.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 34(3):980–91. doi: 10.1523/JNEUROSCI.3725-13.2014.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Lamy, Christophe M., Hitomi Sanno, Gwenaël Labouèbe, Alexandre Picard, Christophe Magnan, Jean Yves Chatton, and Bernard Thorens. 2014. “Hypoglycemia-Activated GLUT2 Neurons of the Nucleus Tractus Solitarius Stimulate Vagal Activity and Glucagon Secretion.” Cell Metabolism 19(3):527–38.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Lange, Wienke, Lei Jin, Vanessa Maybeck, Annika Meisenberg, Arnd Baumann, and Andreas Offenhäusser. 2014. “Recombinant Adeno-Associated Virus (RAAV)-Mediated Transduction and Optogenetic Manipulation of Cortical Neurons in Vitro.” Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics 8928:89282S.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Mortensen, Martin, Favaad Iqbal, Arun P. Pandurangan, Saad Hannan, Rosemary Huckvale, Maya Topf, James R. Baker, and Trevor G. Smart. 2014. “Photo-Antagonism of the GABA A Receptor.” Nature Communications 5.
Equipment used: Flash lamp system (JML-C2) - Norris, Adam D., Shangbang Gao, Megan L. Norris, Debashish Ray, Arun K. Ramani, Andrew G. Fraser, Quaid Morris, Timothy R. Hughes, Mei Zhen, and John A. Calarco. 2014. “A Pair of RNA-Binding Proteins Controls Networks of Splicing Events Contributing to Specialization of Neural Cell Types.” Molecular Cell 54(6):946–59.
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) - Tang, F., S. Lane, A. Korsak, J. F. R. Paton, A. V. Gourine, S. Kasparov, and A. G. Teschemacher. 2014. “Lactate-Mediated Glia-Neuronal Signalling in the Mammalian Brain.” Nature Communications doi: 10.1038/ncomms4284.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) - Wang, Li, Minjie Shen, Yongchun Yu, Yezheng Tao, Ping Zheng, Feifei Wang, and Lan Ma. 2014. “Optogenetic Activation of GABAergic Neurons in the Nucleus Accumbens Decreases the Activity of the Ventral Pallidum and the Expression of Cocaine-Context-Associated Memory.” International Journal of Neuropsychopharmacology 17(5):753–63.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2013
- Couey, Jonathan J., Aree Witoelar, Sheng Jia Zhang, Kang Zheng, Jing Ye, Benjamin Dunn, Rafal Czajkowski, May Britt Moser, Edvard I. Moser, Yasser Roudi, and Menno P. Witter. “Recurrent Inhibitory Circuitry as a Mechanism for Grid Formation.” Nature Neuroscience 16(3):318–24. doi: 10.1038/nn.3310.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Czajkowski, Rafał, Jørgen Sugar, Sheng Jia Zhang, Jonathan J. Couey, Jing Ye, and Menno P. Witter. 2013. “Superficially Projecting Principal Neurons in Layer V of Medial Entorhinal Cortex in the Rat Receive Excitatory Retrosplenial Input.” Journal of Neuroscience 33(40):15779–92. doi: 10.1523/JNEUROSCI.2646-13.2013.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Hägglund, Martin, Kimberly J. Dougherty, Lotta Borgius, Shigeyoshi Itohara, Takuji Iwasato, and Ole Kiehn. 2013. “Optogenetic Dissection Reveals Multiple Rhythmogenic Modules Underlying Locomotion.” Proceedings of the National Academy of Sciences of the United States of America 110(28):11589–94. Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2012
- Threlfell, Sarah, Tatjana Lalic, Nicola J. Platt, Katie A. Jennings, Karl Deisseroth, and Stephanie J. Cragg. 2012. “Striatal Dopamine Release Is Triggered by Synchronized Activity in Cholinergic Interneurons.” Neuron 75(1):58–64. Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473
2011
- Kohl, Michael M., Olivia A. Shipton, Robert M. Deacon, J. Nicholas P. Rawlins, Karl Deisseroth, and Ole Paulsen. 2011. “Hemisphere-Specific Optogenetic Stimulation Reveals Left-Right Asymmetry of Hippocampal Plasticity.” Nature Neuroscience 14(11):1413–15. Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2010
- Bendels, Michael H. K., Prateep Beed, Dietmar Schmitz, Friedrich W. Johenning, and Christian Leibold. 2010. “Detection of Input Sites in Scanning Photostimulation Data Based on Spatial Correlations.” Journal of Neuroscience Methods 192(2):286–95. doi: 10.1016/j.jneumeth.2010.08.006.
Equipment used: Spot illumination module (AiWon) with DPSS 355nm laser 355/30
Photobleaching & FRAP
2023
- Loreau, Vincent, Renate Rees, Eunice Hoyee Chan, Waltraud Taxer, Kathrin Gregor, Bianka Mußil, Christophe Pitaval, Nuno Miguel Luis, Pierre Mangeol, Frank Schnorrer, and Dirk Görlich. “A Nanobody Toolbox to Investigate Localisation and Dynamics of Drosophila Titins and Other Key Sarcomeric Proteins.” ELife 12:1–35. doi: 10.7554/eLife.79343.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with custom laser engine
2022
- Colin‐York, Huw, John Heddleston, Eric Wait, Narain Karedla, Michael DeSantis, Satya Khuon, Teng-leong Chew, Ivo F. Sbalzarini, and Marco Fritzsche. 2022 “Quantifying Molecular Dynamics within Complex Cellular Morphologies Using LLSM‐FRAP.” Small Methods 2200149:2200149. doi: 10.1002/smtd.202200149.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Gruijs da Silva, Lara A., Francesca Simonetti, Saskia Hutten, Henrick Riemenschneider, Erin L. Sternburg, Lisa M. Pietrek, Jakob Gebel, Volker Dötsch, Dieter Edbauer, Gerhard Hummer, Lukas S. Stelzl, and Dorothee Dormann. 2022 “Disease‐linked TDP‐43 Hyperphosphorylation Suppresses TDP‐43 Condensation and Aggregation.” The EMBO Journal. doi: 10.15252/embj.2021108443.
Equipment used: Photomanipulation system UGA-42 Geo with diode single line laser DL-473/75 - Molines, Arthur T., Joël Lemière, Morgan Gazzola, Ida Emilie Steinmark, Claire H. Edrington, Chieh Ting Hsu, Paula Real-Calderon, Klaus Suhling, Gohta Goshima, Liam J. Holt, Manuel Thery, Gary J. Brouhard, and Fred Chang. 2022 “Physical Properties of the Cytoplasm Modulate the Rates of Microtubule Polymerization and Depolymerization.” Developmental Cell 57(4):466-479.e6. doi: 10.1016/j.devcel.2022.02.001.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Trojanowski, Jorge, Lukas Frank, Anne Rademacher, Norbert Mücke, Pranas Grigaitis, and Karsten Rippe. 2022 “Transcription Activation Is Enhanced by Multivalent Interactions Independent of Phase Separation.” Molecular Cell 82(10):1878-1893.e10. doi: 10.1016/j.molcel.2022.04.017.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2021
- Dünkler, Alexander, Marcin Leda, Jan-Michael Kromer, Joachim Neller, Thomas Gronemeyer, Andrew B. Goryachev, and Nils Johnsson. 2021 “Type V Myosin Focuses the Polarisome and Shapes the Tip of Yeast Cells.” Journal of Cell Biology 220 (5): 1–60. https://doi.org/10.1083/jcb.202006193.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-488 - Happle, Andrea, Holger Jeske, and Tatjana Kleinow. 2021. “Dynamic Subcellular Distribution of Begomoviral Nuclear Shuttle and Movement Proteins.” Virology 562:158–75. doi: 10.1016/j.virol.2021.07.014.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-445 and DL-514 - Kimura, Kenichi, Astrid Ooms, Kathrin Graf-Riesen, Maithreyan Kuppusamy, Andreas Unger, Julia Schuld, Jan Daerr, Achim Lother, Caroline Geisen, Lutz Hein, Satoru Takahashi, Guang Li, Wilhelm Röll, Wilhelm Bloch, Peter F. M. van der Ven, Wolfgang A. Linke, Sean M. Wu, Pitter F. Huesgen, Jörg Höhfeld, Dieter O. Fürst, Bernd K. Fleischmann, and Michael Hesse. 2021. “Overexpression of Human BAG3P209L in Mice Causes Restrictive Cardiomyopathy.” Nature Communications 12(1):1–17. doi: 10.1038/s41467-021-23858-7.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Kostan, Julius, Miha Pavšič, Vid Puž, Thomas C. Schwarz, Friedel Drepper, Sibylle Molt, Melissa Ann Graewert, Claudia Schreiner, Sara Sajko, Peter F. M. van der Ven, Adekunle Onipe, Dmitri I. Svergun, Bettina Warscheid, Robert Konrat, Dieter O. Fürst, Brigita Lenarčič, and Kristina Djinović-Carugo. 2021. “Molecular Basis of F-Actin Regulation and Sarcomere Assembly via Myotilin” edited by L. Machesky. PLOS Biology 19(4):e3001148. doi: 10.1371/journal.pbio.3001148.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Lohanadan, Keerthika, Sibylle Molt, Franziska Dierck, Peter F. M. van der Ven, Norbert Frey, Jörg Höhfeld, and Dieter O. Fürst. 2021 “Isoform-Specific Functions of Synaptopodin-2 Variants in Cytoskeleton Stabilization and Autophagy Regulation in Muscle under Mechanical Stress.” Experimental Cell Research 408(2):112865. doi: 10.1016/j.yexcr.2021.112865.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Malavasi, Elise LV, Aniket Ghosh, Daniel G. Booth, Michele Zagnoni, Diane L. Sherman, and Peter J. Brophy. 2021. “Dynamic Early Clusters of Nodal Proteins Contribute to Node of Ranvier Assembly during Myelination of Peripheral Neurons.” ELife 10:1–29. doi: 10.7554/elife.68089.
Equipment used: Photomanipulation system UGA-40 Firefly and UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-405 and DL-473 - Naffa, Randa, Rita Padányi, Attila Ignácz, Zoltán Hegyi, Bálint Jezsó, Sarolta Tóth, Karolina Varga, László Homolya, Luca Hegedűs, Katalin Schlett, and Agnes Enyedi. 2021 “The Plasma Membrane Ca2+ Pump Pmca4b Regulates Melanoma Cell Migration through Remodeling of the Actin Cytoskeleton.” Cancers 13(6):1–25. doi: 10.3390/cancers13061354.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-488 - Oueslati Morales, Carlos O., Attila Ignácz, Norbert Bencsik, Zsofia Sziber, Anikó Erika Rátkai, Wolfgang S. Lieb, Stephan A. Eisler, Attila Szűcs, Katalin Schlett, and Angelika Hausser. 2021 “Protein Kinase D Promotes Activity‐dependent AMPA Receptor Endocytosis in Hippocampal Neurons.” Traffic 22(12):454–70. doi: 10.1111/tra.12819.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Peng GE, Pessino V, Huang B, von Zastrow M (2021) Spatial decoding of endosomal cAMP signals by a metastable cytoplasmic PKA network. Nat Chem Biol Available at: http://www.nature.com/articles/s41589-021-00747-0.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Pitzen, Valentin, Sophia Sander, Otto Baumann, Ralph Gräf, and Irene Meyer. 2021 “Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae.” Cells 10(9):2384. doi: 10.3390/cells10092384.
Equipment used: Photomanipulation system UGA-40 Firefly and UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-405 and DL-473 - Pollak, Nadine, Aline Lindner, Dirke Imig, Karsten Kuritz, Jacques S. Fritze, Lorena Decker, Isabel Heinrich, Jannis Stadager, Stephan Eisler, Daniela Stöhr, Frank Allgöwer, Peter Scheurich, and Markus Rehm. 2021 “Cell Cycle Progression and Transmitotic Apoptosis Resistance Promote Escape from Extrinsic Apoptosis.” Journal of Cell Science. doi: 10.1242/jcs.258966.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-515 - Riegert, Janine, Alexander Töpel, Jana Schieren, Renee Coryn, Stella Dibenedetto, Dominik Braunmiller, Kamil Zajt, Carmen Schalla, Stephan Rütten, Martin Zenke, Andrij Pich, and Antonio Sechi. 2021 “Guiding Cell Adhesion and Motility by Modulating Cross-Linking and Topographic Properties of Microgel Arrays.” Plos One 16(9):e0257495. doi: 10.1371/journal.pone.0257495.
Equipment used: Photomanipulation system UGA-40 Firefly and UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-405. - Schäringer, Katja, Sebastian Maxeiner, Carmen Schalla, Stephan Rütten, Martin Zenke, and Antonio Sechi. 2021 “LSP1-Myosin1e Bimolecular Complex Regulates Focal Adhesion Dynamics and Cell Migration.” FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology 35(2):e21268. doi: 10.1096/fj.202000740RR.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Schmidt, Anja, Long Li, Zhiyi Lv, Shuling Yan, and Jörg Großhans. 2021. “Dia- and Rok-Dependent Enrichment of Capping Proteins in a Cortical Region.” Journal of Cell Science. doi: 10.1242/jcs.258973.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Tulpule, Asmin, Juan Guan, Dana S. Neel, Hannah R. Allegakoen, Yone Phar Lin, David Brown, Yu-Ting Chou, Ann Heslin, Nilanjana Chatterjee, Shriya Perati, Shruti Menon, Tan A. Nguyen, Jayanta Debnath, Alejandro D. Ramirez, Xiaoyu Shi, Bin Yang, Siyu Feng, Suraj Makhija, Bo Huang, and Trever G. Bivona. 2021 “Kinase-Mediated RAS Signaling via Membraneless Cytoplasmic Protein Granules.” Cell 184(10):2649-2664.e18. doi: 10.1016/j.cell.2021.03.031.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-473 - Wang, Mengzhe, Tatjana Kleele, Yan Xiao, Gabriela Plucinska, Petros Avramopoulos, Stefan Engelhardt, Markus H. Schwab, Matthias Kneussel, Tim Czopka, Diane L. Sherman, Peter J. Brophy, Thomas Misgeld, and Monika S. Brill. “Completion of Neuronal Remodeling Prompts Myelination along Developing Motor Axon Branches.” Journal of Cell Biology 220(4). doi: 10.1083/JCB.201911114.
Equipment used: Photomanipulation system UGA-42 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Zisis, Themistoklis, Jan Schwarz, Miriam Balles, Maibritt Kretschmer, Maria Nemethova, Remy Chait, Robert Hauschild, Janina Lange, Calin Guet, Michael Sixt, and Stefan Zahler. 2021. “Sequential and Switchable Patterning for Studying Cellular Processes under Spatiotemporal Control.” ACS Applied Materials & Interfaces 13(30):35545–60. doi: 10.1021/acsami.1c09850.
Equipment used: customized upward-facing collimated LED systems (see LED illumination systems)
2020
- Belyy, Vladislav, Ngoc Han Tran, and Peter Walter. 2020. “Quantitative Microscopy Reveals Dynamics and Fate of Clustered IRE1α. ” Proceedings of the National Academy of Sciences of the United States of America 117(3):1533–42.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Djinovic-Carugo, Kristina and Julius Kostan. 2020. “Structural Insights into F-Actin Regulation and Sarcomere Assembly via Myotilin.” Biophysical Journal 118(3):495a.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Ghosh, Aniket, Elise Lv Malavasi, Diane L. Sherman, and Peter J. Brophy. 2020. “Neurofascin and Kv7.3 Are Delivered to Somatic and Axon Terminal Surface Membranes En Route to the Axon Initial Segment.” ELife 9:1–17.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-405 and DL-473 - Grinhagens, Sören, Alexander Dünkler, Yehui Wu, Lucia Rieger, Philipp Brenner, Thomas Gronemeyer, Medhanie A. Mulaw, and Nils Johnsson. 2020. “A Time-Resolved Interaction Analysis of Bem1 Reconstructs the Flow of Cdc42 during Polar Growth.” Life Science Alliance 3(9):1–16.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-488 - Hutten, Saskia, Sinem Usluer, Benjamin Bourgeois, Francesca Simonetti, Hana M. Odeh, Charlotte M. Fare, Mareike Czuppa, Marian Hruska-Plochan, Mario Hofweber, Magdalini Polymenidou, James Shorter, Dieter Edbauer, Tobias Madl, and Dorothee Dormann. 2020 “Nuclear Import Receptors Directly Bind to Arginine-Rich Dipeptide Repeat Proteins and Suppress Their Pathological Interactions.” Cell Reports 33(12):108538.
Equipment used: Photomanipulation system UGA-42 Geo with diode single line laser DL-473 - Kleinow, Tatjana, Andrea Happle, Sigrid Kober, Luise Linzmeier, Tina M. Rehm, Jacques Fritze, Patrick C. F. Buchholz, Gabi Kepp, Holger Jeske, and Christina Wege. 2020. “Phosphorylations of the Abutilon Mosaic Virus Movement Protein Affect Its Self-Interaction, Symptom Development, Viral DNA Accumulation, and Host Range.” Frontiers in Plant Science 11(July):1–20.
Equipment used: Photomanipulation system (UGA-42 Firefly) with diode single line laser DL-515 - Koch, Nicole, Dennis Koch, Sarah Krueger, Jessica Tröger, Victor Sabanov, Tariq Ahmed, Laura E. McMillan, David Wolf, Dirk Montag, Michael M. Kessels, Detlef Balschun, and Britta Qualmann. 2020. “Syndapin I Loss-of-Function in Mice Leads to Schizophrenia-Like Symptoms.” Cerebral Cortex 1–19. Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
- Passmore, Josiah B., Ruth E. Carmichael, Tina A. Schrader, Luis F. Godinho, Sacha Ferdinandusse, Celien Lismont, Yunhong Wang, Christian Hacker, Markus Islinger, Marc Fransen, David M. Richards, Peter Freisinger, and Michael Schrader. 2020. “Mitochondrial Fission Factor (MFF) Is a Critical Regulator of Peroxisome Maturation.” Biochimica et Biophysica Acta (BBA) – Molecular Cell Research 118709.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Pauls, Samantha D., Sen Hou, and Aaron J. Marshall. 2020. “SHIP Interacts with Adaptor Protein Nck and Restricts Actin Turnover in B Cells.” Biochemical and Biophysical Research Communications 527(1):207–12.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Steinberg, Gero, Martin Schuster, Sarah J. Gurr, Tina A. Schrader, Michael Schrader, Mark Wood, Andy Early, and Sreedhar Kilaru. 2020. “A Lipophilic Cation Protects Crops against Fungal Pathogens by Multiple Modes of Action.” Nature Communications 11(1). Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405
- von Appen, Alexander, Dollie LaJoie, Isabel E. Johnson, Michael J. Trnka, Sarah M. Pick, Alma L. Burlingame, Katharine S. Ullman, and Adam Frost. 2020. “LEM2 Phase Separation Promotes ESCRT-Mediated Nuclear Envelope Reformation.” Nature. 582(7810):115–18.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2019
- Batsios, Petros, Hellen C. Ishikawa-Ankerhold, Heike Roth, Michael Schleicher, Catherine C. L. Wong, and Annette Müller-Taubenberger. 2019. “Ate1-Mediated Posttranslational Arginylation Affects Substrate Adhesion and Cell Migration in Dictyostelium Discoideum.” Molecular Biology of the Cell 30(4):453–66.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Feng, Siyu, Aruna Varshney, Doris Coto Villa, Cyrus Modavi, John Kohler, Fatima Farah, Shuqin Zhou, Nebat Ali, Joachim D. Müller, Miri K. Van Hoven, and Bo Huang. 2019. “Bright Split Red Fluorescent Proteins for the Visualization of Endogenous Proteins and Synapses.” Communications Biology 2(1):1–12.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Skamrahl, Mark, Huw Colin-York, Liliana Barbieri, and Marco Fritzsche. 2019. “Simultaneous Quantification of the Interplay Between Molecular Turnover and Cell Mechanics by AFM–FRAP.” Small 15(40):1–9.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-488
2018
- Ederle, Helena, Christina Funk, Claudia Abou-Ajram, Saskia Hutten, Eva B. E. Funk, Ralph H. Kehlenbach, Susanne M. Bailer, and Dorothee Dormann. 2018. “Nuclear Egress of TDP-43 and FUS Occurs Independently of Exportin-1/CRM1.” Scientific Reports 8(1):1–18.
Equipment used: Photomanipulation system UGA-42 Firefly with diode single line laser DL-473 - Eichel, Kelsie, Damien Jullié, Benjamin Barsi-Rhyne, Naomi R. Latorraca, Matthieu Masureel, Jean Baptiste Sibarita, Ron O. Dror, and Mark Von Zastrow. 2018. “Catalytic Activation of β-Arrestin by GPCRs.” Nature 557(7705):381–86.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Hofweber, Mario, Saskia Hutten, Benjamin Bourgeois, Emil Spreitzer, Annika Niedner-Boblenz, Martina Schifferer, Marc David Ruepp, Mikael Simons, Dierk Niessing, Tobias Madl, and Dorothee Dormann. 2018. “Phase Separation of FUS Is Suppressed by Its Nuclear Import Receptor and Arginine Methylation.” Cell 173(3):706-719.e13.
Equipment used: Photomanipulation system UGA-42 Geo with diode single line laser DL-473 - Ghosh Moulick, Ranjita, Gregor Panaitov, Sung-Eun Choi, Dirk Mayer, and Andreas Offenhäusser. 2018. “Patterning Artificial Lipid Bilayer on Nanostructured Surfaces.” International Journal of Nanomedicine Volume 13:55–58. doi: 10.2147/IJN.S125005.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Pitzen, Valentin, Sophie Askarzada, Ralph Gräf, and Irene Meyer. 2018. “CDK5RAP2 Is an Essential Scaffolding Protein of the Corona of the Dictyostelium Centrosome.” Cells 7(4):32.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Stoeber, Miriam, Damien Jullié, Braden T. Lobingier, Toon Laeremans, Jan Steyaert, Peter W. Schiller, Aashish Manglik, and Mark von Zastrow. 2018. “A Genetically Encoded Biosensor Reveals Location Bias of Opioid Drug Action.” Neuron 98(5):963-976.e5.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2017
- Carbone, Catherine B., Nadja Kern, Ricardo A. Fernandes, Enfu Hui, Xiaolei Su, K. Christopher Garcia, and Ronald D. Vale. 2017. “In Vitro Reconstitution of T Cell Receptor-Mediated Segregation of the CD45 Phosphatase.” Proceedings of the National Academy of Sciences of the United States of America 114(44):E9338–45.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Dierck, Franziska, Christian Kuhn, Claudia Rohr, Susanne Hille, Julia Braune, Samuel Sossalla, Sibylle Molt, Peter F. M. Van Der Ven, Dieter O. Fürst, and Norbert Frey. 2017. “The Novel Cardiac Z-Disc Protein CEFIP Regulates Cardiomyocyte Hypertrophy by Modulating Calcineurin Signaling.” Journal of Biological Chemistry 292(37):15180–91.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Dreser, Alice, Jan Tilmann Vollrath, Antonio Sechi, Sonja Johann, Andreas Roos, Alfred Yamoah, Istvan Katona, Saeed Bohlega, Dominik Wiemuth, Yuemin Tian, Axel Schmidt, Jörg Vervoorts, Marc Dohmen, Cordian Beyer, Jasper Anink, Eleonora Aronica, Dirk Troost, Joachim Weis, and Anand Goswami. 2017. “The ALS-Linked E102Q Mutation in Sigma Receptor-1 Leads to ER Stress-Mediated Defects in Protein Homeostasis and Dysregulation of RNA-Binding Proteins.” Cell Death and Differentiation 24(10):1655–71.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Guimarães, Sofia C., Sreedhar Kilaru, Michael Schrader, and Martin Schuster. 2017. “Labeling of Peroxisomes for Live Cell Imaging in the Filamentous Fungus Ustilago Maydis.” Pp. 131–50 in Methods in Molecular Biology. Vol. 1595.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Jain, Ankur and Ronald D. Vale. 2017. “RNA Phase Transitions in Repeat Expansion Disorders.” Nature 546(7657):243–47.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Reimann, Lena, Heike Wiese, Yvonne Leber, Anja N. Schwable, Anna L. Fricke, Anne Rohland, Bettina Knapp, Christian D. Peikert, Friedel Drepper, Peter F. M. Van Der Ven, Gerald Radziwill, Dieter O. Furst, and Bettina Warscheid. 2017. “Myofibrillar Z-Discs Are a Protein Phosphorylation Hot Spot with Protein Kinase C (PKCα) Modulating Protein Dynamics.” Molecular and Cellular Proteomics 16(3):346–67.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2016
- Batsios, Petros, Xiang Ren, Otto Baumann, Denis Larochelle, and Ralph Gräf. 2016. “Src1 Is a Protein of the Inner Nuclear Membrane Interacting with the Dictyostelium Lamin NE81.” Cells 5(1):13.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Eichel, K., D. Jullié, and M. Von Zastrow. 2016. “β-Arrestin Drives MAP Kinase Signalling from Clathrin-Coated Structures after GPCR Dissociation.” Nature Cell Biology 18(3):303–10.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Leber, Yvonne, Avnika A. Ruparelia, Gregor Kirfel, Peter F. M. van der Ven, Bernd Hoffmann, Rudolf Merkel, Robert J. Bryson-Richardson, and Dieter O. Fürst. 2016. “Filamin C Is a Highly Dynamic Protein Associated with Fast Repair of Myofibrillar Microdamage.” Human Molecular Genetics 25(13):2776–88.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Lin, Congping, Martin Schuster, Sofia Cunha Guimaraes, Peter Ashwin, Michael Schrader, Jeremy Metz, Christian Hacker, Sarah Jane Gurr, and Gero Steinberg. 2016. “Active Diffusion and Microtubule-Based Transport Oppose Myosin Forces to Position Organelles in Cells.” Nature Communications 7(1):11814.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Pauls, Samantha D., Arnab Ray, Sen Hou, Andrew T. Vaughan, Mark S. Cragg, and Aaron J. Marshall. 2016. “FcγRIIB-Independent Mechanisms Controlling Membrane Localization of the Inhibitory Phosphatase SHIP in Human B Cells.” The Journal of Immunology 197(5):1587–96.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-405 - Putzler, Sascha, Irene Meyer, and Ralph Gräf. 2016. “CP91 Is a Component of the Dictyostelium Centrosome Involved in Centrosome Biogenesis.” European Journal of Cell Biology 95(3–5):124–35.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473 - Voigt, Andreas, Romy Freund, Jennifer Heck, Markus Missler, Gerald J. Obermair, Ulrich Thomas, and Martin Heine. 2016. “Dynamic Association of Calcium Channel Subunits at the Cellular Membrane.” Neurophotonics 3(4):041809. doi: 10.1117/1.nph.3.4.041809. Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2015
- Guimaraes, Sofia C., Martin Schuster, Ewa Bielska, Gulay Dagdas, Sreedhar Kilaru, Ben R. A. Meadows, Michael Schrader, and Gero Steinberg. 2015. “Peroxisomes, Lipid Droplets, and Endoplasmic Reticulum ‘Hitchhike’ on Motile Early Endosomes.” Journal of Cell Biology 211(5):945–54.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-405 - Lee, Kyung Jong, Janapriya Saha, Jingxin Sun, Kazi R. Fattah, Shu Chi Wang, Burkhard Jakob, Linfeng Chi, Shih Ya Wang, Gisela Taucher-Scholz, Anthony J. Davis, and David J. Chen. 2015. “Phosphorylation of Ku Dictates DNA Double-Strand Break (DSB) Repair Pathway Choice in S Phase.” Nucleic Acids Research 44(4):1732–45.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-473 - Neupert, Christian, Romy Schneider, Oliver Klatt, Carsten Reissner, Daniele Repetto, Barbara Biermann, Katharina Niesmann, Markus Missler, and Martin Heine. 2015. “Regulated Dynamic Trafficking of Neurexins inside and Outside of Synaptic Terminals.” Journal of Neuroscience 35(40):13629–47.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2014
- Cijsouw, Tony, Jens P. Weber, Jurjen H. Broeke, Jantine A. C. Broek, Desiree Schut, Tim Kroon, Ingrid Saarloos, Matthijs Verhage, and Ruud F. Toonen. 2014. “Munc18-1 Redistributes in Nerve Terminals in an Activity- and PKC-Dependent Manner.” Journal of Cell Biology 204(5):759–75. Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2013
- Fernandez-Fernandez, Carmen, Karin Grosse, Victor Sourjik, and Justine Collier. 2013. “The β-Sliding Clamp Directs the Localization of HdaA to the Replisome in Caulobacter Crescentus.” Microbiology (United Kingdom) 159(PART11):2237–48. doi: 10.1099/mic.0.068577-0.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-532 - Press, Maximilian O., Hui Li, Nicole Creanza, Günter Kramer, Christine Queitsch, Victor Sourjik, and Elhanan Borenstein. 2013. “Genome-Scale Co-Evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90” edited by I. Matic. PLoS Genetics 9(7):e1003631. doi: 10.1371/journal.pgen.1003631.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-532
2012
- Kuhnert, Oliver, Otto Baumann, Irene Meyer, and Ralph Gräf. 2012. “CP55, a Novel Key Component of Centrosomal Organization in Dictyostelium.” Cellular and Molecular Life Sciences : CMLS 69(21):3651–64.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2010
- Küpper, Kevin, Nadine Lang, Christoph Möhl, Norbert Kirchgessner, Simone Born, Wolfgang H. Goldmann, Rudolf Merkel, and Bernd Hoffmann. 2010. “Tyrosine Phosphorylation of Vinculin at Position 1065 Modifies Focal Adhesion Dynamics and Cell Tractions.” Biochemical and Biophysical Research Communications 399(4):560–64.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with diode single line laser DL-473
2009
- Kentner, David, and Victor Sourjik. “Dynamic Map of Protein Interactions in the Escherichia Coli Chemotaxis Pathway.” Molecular Systems Biology 5(238). doi: 10.1038/msb.2008.77.
Equipment used: Spot illumination module (AiWon) with diode single line laser DL-532
2007
- Weiskopf, Daniela, Eva K. Schmitt, Marco H. Klühr, Stephan K. Dertinger, and Claudia Steinem. 2007. “Micro-BLMs on Highly Ordered Porous Silicon Substrates: Rupture Process and Lateral Mobility.” Langmuir : The ACS Journal of Surfaces and Colloids 23(18):9134–39.
Equipment used: Spot illumination module (AiWon)
Multi-Photon Imaging
2023
- Danyukova, Tatyana, Assil-Ramin Alimy, Renata Voltolini Velho, Timur A. Yorgan, Giorgia Di Lorenzo, Simon von Kroge, Henning Tidow, J. Simon Wiegert, Irm Hermans-Borgmeyer, Thorsten Schinke, Tim Rolvien, and Sandra Pohl. 2023. “Mice Heterozygous for an Osteogenesis Imperfecta-Linked MBTPS2 Variant Display a Compromised Subchondral Osteocyte Lacunocanalicular Network Associated with Abnormal Articular Cartilage.” Bone 177:116927. doi: 10.1016/j.bone.2023.116927.
Equipment used: multiphoton scanning microscope (DF-scope from Sutter Instrument), custom modified by Rapp OptoElectronic
2022
- Fieblinger, Tim, Alberto Perez‐Alvarez, Paul J. Lamothe‐Molina, Christine E. Gee, and Thomas G. Oertner. 2022. “Presynaptic CGMP Sets Synaptic Strength in the Striatum and Is Important for Motor Learning.” EMBO Reports. doi: 10.15252/embr.202154361.
Equipment used: large field of view multiphoton scanning microscope (Rapid3Dscope) from Rapp OptoElectronic.
2021
- Perez-Alvarez, Alberto, Florian Huhn, Céline D. Dürst, Andreas Franzelin, Paul J. Lamothe-Molina, and Thomas G. Oertner. 2021. “Freeze-Frame Imaging of Dendritic Calcium Signals With TubuTag.” Frontiers in Molecular Neuroscience 14:1–14. doi: 10.3389/fnmol.2021.635820.
Equipment used: large field of view multiphoton scanning microscope Rapid3Dscope from Rapp OptoElectronic. - Yang, Wei, Mattia Chini, Jastyn A. Pöpplau, Andrey Formozov, Alexander Dieter, Patrick Piechocinski, Cynthia Rais, Fabio Morellini, Olaf Sporns, Ileana L. Hanganu-Opatz, and J. Simon Wiegert. “Anesthetics Fragment Hippocampal Network Activity, Alter Spine Dynamics, and Affect Memory Consolidation” edited by J. Csicsvari. PLOS Biology 19(4):e3001146. doi: 10.1371/journal.pbio.3001146.
Equipment used: multiphoton scanning microscope (movable objective microscope MOM from Sutter Instrument), custom modified by Rapp OptoElectronic.
2020
- Perez-Alvarez, Alberto, Brenna C. Fearey, Ryan J. O’Toole, Wei Yang, Ignacio Arganda-Carreras, Paul J. Lamothe-Molina, Benjamien Moeyaert, Manuel A. Mohr, Lauren C. Panzera, Christian Schulze, Eric R. Schreiter, J. Simon Wiegert, Christine E. Gee, Michael B. Hoppa, and Thomas G. Oertner. “Freeze-Frame Imaging of Synaptic Activity Using SynTagMA.” Nature Communications 11(1):2464. doi: 10.1038/s41467-020-16315-4.
Equipment used: multiphoton scanning microscope (movable objective microscope MOM from Sutter Instrument), custom modified by Rapp OptoElectronic. - Westermann, Lena Marie, Lutz Fleischhauer, Jonas Vogel, Zsuzsa Jenei-Lanzl, Nataniel Floriano Ludwig, Lynn Schau, Fabio Morellini, Anke Baranowsky, Timur A. Yorgan, Giorgia Di Lorenzo, Michaela Schweizer, Bruna de Souza Pinheiro, Nicole Ruas Guarany, Fernanda Sperb-Ludwig, Fernanda Visioli, Thiago Oliveira Silva, Jamie Soul, Gretl Hendrickx, J. Simon Wiegert, Ida V. D. Schwartz, Hauke Clausen-Schaumann, Frank Zaucke, Thorsten Schinke, Sandra Pohl, and Tatyana Danyukova. “Imbalanced Cellular Metabolism Compromises Cartilage Homeostasis and Joint Function in a Mouse Model of Mucolipidosis Type III Gamma.” Disease Models & Mechanisms 13(11):dmm046425. doi: 10.1242/dmm.046425.
Equipment used: multiphoton scanning microscope (DF-scope from Sutter Instrument), custom modified by Rapp OptoElectronic
2018
- Oda, Kazumasa, Johannes Vierock, Satomi Oishi, Silvia Rodriguez-Rozada, Reiya Taniguchi, Keitaro Yamashita, J. Simon Wiegert, Tomohiro Nishizawa, Peter Hegemann, and Osamu Nureki. “Crystal Structure of the Red Light-Activated Channelrhodopsin Chrimson.” Nature Communications 9(1):3949. doi: 10.1038/s41467-018-06421-9.
Equipment used: multiphoton scanning microscope (DF-scope from Sutter Instrument), custom modified by Rapp OptoElectronic
2016
- Fichte, Manuela A. H., Xenia M. M. Weyel, Stephan Junek, Florian Schäfer, Cyril Herbivo, Maurice Goeldner, Alexandre Specht, Josef Wachtveitl, and Alexander Heckel. 2016 “Three-Dimensional Control of DNA Hybridization by Orthogonal Two-Color Two-Photon Uncaging.” Angewandte Chemie International Edition 55(31):8948–52. doi: 10.1002/anie.201603281.
Equipment used: Photomanipulation system UGA-40 Firefly (UGA-42 Firefly) with multiphoton laser
Microscope Illumination
2023
- Choudhary, Sakshi, Gefen Livne, Shachar Gat, and Anne Bernheim-Groswasser. “The Mechanics of (Poro-)Elastic Contractile Actomyosin Networks As a Model System of the Cell Cytoskeleton.” Journal of Visualized Experiments (193):2–3. doi: 10.3791/64377.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) - Pizzoni, Alejandro, Xuefeng Zhang, Nyla Naim, and Daniel L. Altschuler. 2023. “Soluble Cyclase-Mediated Nuclear CAMP Synthesis Is Sufficient for Cell Proliferation.” Proceedings of the National Academy of Sciences 120(4):1–22. doi: 10.1073/pnas.2208749120.
Equipment used: IX3-Breadboard - Rohrbeck, Matthias, Verena Hoerr, Ilaria Piccini, Boris Greber, Jan Sebastian Schulte, Sara-sophie Hübner, Elena Jeworutzki, Carsten Theiss, Veronika Matschke, Jörg Stypmann, Andreas Unger, Huyen Tran Ho, Paul Disse, Nathalie Strutz-Seebohm, Cornelius Faber, Frank Ulrich Müller, Stephan Ludwig, Ursula Rescher, Wolfgang A. Linke, Karin Klingel, Karin Busch, Stefan Peischard, and Guiscard Seebohm. 2023. “Pathophysiological Mechanisms of Cardiac Dysfunction in Transgenic Mice with Viral Myocarditis.” Cells 12(4):550. doi: 10.3390/cells12040550.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Stöberl, Stefan, Miriam Balles, Thomas Kellerer, and Joachim O. Rädler. “Photolithographic Microfabrication of Hydrogel Clefts for Cell Invasion Studies.” Lab on a Chip 23(7):1886–95. doi: 10.1039/D2LC01105K.
Equipment used: Custom LED illumination - Tacke, Charlotte, Anne M. Bischoff, Ali Harb, Behnam Vafadari, and Swen Hülsmann. “Fiber Optical Imaging of Astroglial Calcium Signaling in the Respiratory Network in the Working Heart Brainstem Preparation.” Frontiers in Physiology 14(August):1–7. doi: 10.3389/fphys.2023.1237376.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2)
2022
- Engel, Marcial Alexander, Yves René Wörmann, Hanna Kaestner, and Christina Schüler. 2022. “An Optogenetic Arrhythmia Model—Insertion of Several Catecholaminergic Polymorphic Ventricular Tachycardia Mutations Into Caenorhabditis Elegans UNC-68 Disturbs Calstabin-Mediated Stabilization of the Ryanodine Receptor Homolog.” Frontiers in Physiology 13(March). doi: 10.3389/fphys.2022.691829.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Hirschberg, Stefan, Anton Dvorzhak, Seyed M. A. Rasooli-Nejad, Svilen Angelov, Marieluise Kirchner, Philipp Mertins, Gilla Lättig-Tünnemann, Christoph Harms, Dietmar Schmitz, and Rosemarie Grantyn 2022. “Uncoupling the Excitatory Amino Acid Transporter 2 From Its C-Terminal Interactome Restores Synaptic Glutamate Clearance at Corticostriatal Synapses and Alleviates Mutant Huntingtin-Induced Hypokinesia.” Frontiers in Cellular Neuroscience 15(January):1–23. doi: 10.3389/fncel.2021.792652.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Sperandeo, Alessandra, Claudia Tamburini, Zoe Noakes, Daniel Cabezas de la Fuente, Francesca Keefe, Olena Petter, William Plumbly, Nicholas Clifton, Meng Li, and Kathryn Peall. 2022. “Cortical Neuronal Hyperexcitability and Synaptic Changes in SGCE Mutation-Positive Myoclonus Dystonia.” Brain. doi: 10.1093/brain/awac365.
Equipment used: single wavelength LED Illumination system - Vettkötter, Dennis, Martin Schneider, Brady D. Goulden, Holger Dill, Jana Liewald, Sandra Zeiler, Julia Guldan, Yilmaz Arda Ateş, Shigeki Watanabe, and Alexander Gottschalk. “Rapid and Reversible Optogenetic Silencing of Synaptic Transmission by Clustering of Synaptic Vesicles.” Nature Communications 13(1). doi: 10.1038/s41467-022-35324-z.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm
2021
- Amini, Mahnaz, Yiming Chang, Ulrich Wissenbach, Veit Flockerzi, Gabriel Schlenstedt, and Andreas Beck. 2021. “Activity of the Yeast Vacuolar TRP Channel TRPY1 Is Inhibited by Ca2+–Calmodulin Binding.” Journal of Biological Chemistry 297(4):101126. doi: 10.1016/j.jbc.2021.101126.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Anni, Daniela, Eva-Maria Weiss, Debarpan Guhathakurta, Yagiz Enes Akdas, Julia Klueva, Stefanie Zeitler, Maria Andres-Alonso, Tobias Huth, and Anna Fejtova. 2021. “Aβ1-16 Controls Synaptic Vesicle Pools at Excitatory Synapses via Cholinergic Modulation of Synapsin Phosphorylation.” Cellular and Molecular Life Sciences (0123456789). doi: 10.1007/s00018-021-03835-5.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Bakr, May, Damien Jullié, Julia Krapivkina, Vincent Paget-Blanc, Lou Bouit, Jennifer D. Petersen, Natacha Retailleau, Christelle Breillat, Etienne Herzog, Daniel Choquet, and David Perrais. 2021. “The VSNAREs VAMP2 and VAMP4 Control Recycling and Intracellular Sorting of Post-Synaptic Receptors in Neuronal Dendrites.” Cell Reports 36(10):109678. doi: 10.1016/j.celrep.2021.109678.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm
2020
- Oyabu, Kohei, Kotomi Takeda, Hiroyuki Kawano, Kaori Kubota, Takuya Watanabe, N. Charles Harata, Shutaro Katsurabayashi, and Katsunori Iwasaki. 2020. “Presynaptically Silent Synapses Are Modulated by the Density of Surrounding Astrocytes.” Journal of Pharmacological Sciences 144(2):76–82. doi: 10.1016/j.jphs.2020.07.009.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 365 nm
2019
- Bakooshli, Mohsen Afshar, Ethan S. Lippmann, Ben Mulcahy, Nisha Iyer, Christine T. Nguyen, Kayee Tung, Bryan A. Stewart, Hubrecht Van Den Dorpel, Tobias Fuehrmann, Molly Shoichet, Anne Bigot, Elena Pegoraro, Henry Ahn, Howard Ginsberg, Mei Zhen, Randolph Scott Ashton, and Penney M. Gilbert. 2019. “A 3d Culture Model of Innervated Human Skeletal Muscle Enables Studies of the Adult Neuromuscular Junction.” ELife 8:1–29. doi: 10.7554/eLife.44530.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Caluori, Guido, Roberto Raiteri, and Mariateresa Tedesco. 2019. “Simultaneous AFM Investigation of the Single Cardiomyocyte Electro-Chemo-Mechanics During Excitation-Contraction Coupling.” Pp. 355–67 in Methods in Molecular Biology.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Hülsmann, Swen, Yoshihiko Oke, Guillaume Mesuret, A. Tobias Latal, Michal G. Fortuna, Marcus Niebert, Johannes Hirrlinger, Julia Fischer, and Kurt Hammerschmidt. 2019. “The Postnatal Development of Ultrasonic Vocalization-Associated Breathing Is Altered in Glycine Transporter 2-Deficient Mice.” Journal of Physiology 597(1):173–91. doi: 10.1113/JP276976.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Woldemariam, Sarah, Jatin Nagpal, Tyler Hill, Joy Li, Martin W. Schneider, Raakhee Shankar, Mary Futey, Aruna Varshney, Nebat Ali, Jordan Mitchell, Kristine Andersen, Benjamin Barsi-Rhyne, Alan Tran, Wagner Steuer Costa, Michelle C. Krzyzanowski, Yanxun V. Yu, Chantal Brueggemann, O. Scott Hamilton, Denise M. Ferkey, Miri VanHoven, Piali Sengupta, Alexander Gottschalk, and Noelle L’Etoile. 2019 “Using a Robust and Sensitive GFP-Based CGMP Sensor for Real-Time Imaging in Intact Caenorhabditis Elegans.” Genetics 213(1):59–77. doi: 10.1534/genetics.119.302392.3
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) with 470 and 590 nm
2018
- Dietrich, Miriam, Hugo Le Roy, David B. Brückner, Hanna Engelke, Roman Zantl, Joachim O. Rädler, and Chase P. Broedersz. 2018. “Guiding 3D Cell Migration in Deformed Synthetic Hydrogel Microstructures.” Soft Matter 14(15):2816–26. doi: 10.1039/C8SM00018B.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 365 nm - Koblinger, Kathrin, Céline Jean-Xavier, Sandeep Sharma, Tamás Füzesi, Leanne Young, Shane E. A. Eaton, Charlie Hong Ting Kwok, Jaideep Singh Bains, and Patrick J. Whelan. 2018. “Optogenetic Activation of A11 Region Increases Motor Activity.” Frontiers in Neural Circuits 12(October):1–15. doi: 10.3389/fncir.2018.00086.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Leitner, Michael G., Kirstin Hobiger, Angeliki Mavrantoni, Anja Feuer, Johannes Oberwinkler, Dominik Oliver, and Christian R. Halaszovich. 2018. “A126 in the Active Site and TI167/168 in the TI Loop Are Essential Determinants of the Substrate Specificity of PTEN.” Cellular and Molecular Life Sciences 75(22):4235–50. doi: 10.1007/s00018-018-2867-z.
Equipment used: TIRF condenser - Strigli, Anne, Christian Raab, Sabine Hessler, Tobias Huth, Adam J. T. Schuldt, Christian Alzheimer, Thomas Friedrich, Paul W. Burridge, Mark Luedde, and Michael Schwake. 2018. “Doxorubicin Induces Caspase-Mediated Proteolysis of KV7.1.” Communications Biology 1(1):155.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Tolstenkov, Oleg, Petrus Van der Auwera, Wagner Steuer Costa, Olga Bazhanova, Tim M. Gemeinhardt, Amelie C. F. Bergs, and Alexander Gottschalk. 2018. “Functionally Asymmetric Motor Neurons Contribute to Coordinating Locomotion of Caenorhabditis Elegans.” ELife 7:1–28. doi: 10.7554/eLife.34997.
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) with 470 and 590 nm - Vickers, Evan D., Christopher Clark, Denys Osypenko, Alex Fratzl, Olexiy Kochubey, Bernhard Bettler, and Ralf Schneggenburger. 2018. “Parvalbumin-Interneuron Output Synapses Show Spike-Timing-Dependent Plasticity That Contributes to Auditory Map Remodeling.” Neuron 99(4):720-735.e6. doi: 10.1016/j.neuron.2018.07.018.
Equipment used: Microscope illumination coupling
2017
- Beck, Andreas, Viktoria Götz, Sen Qiao, Petra Weissgerber, Veit Flockerzi, Marc Freichel, and Ulrich Boehm. 2017. “Functional Characterization of Transient Receptor Potential (TRP) Channel C5 in Female Murine Gonadotropes.” Endocrinology 158(4):887–902. doi: 10.1210/en.2016-1810.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Thiriot, Aude, Carolina Perdomo, Guiying Cheng, Igor Novitzky-Basso, Sara McArdle, Jamie K. Kishimoto, Olga Barreiro, Irina Mazo, Robinson Triboulet, Klaus Ley, Antal Rot, and Ulrich H. von Andrian. 2017. “Differential DARC/ACKR1 Expression Distinguishes Venular from Non-Venular Endothelial Cells in Murine Tissues.” BMC Biology 15(1):45. doi: 10.1186/s12915-017-0381-7.
Equipment used: Flash lamp system SP-20
2016
- Bennke, Christin M., Greta Reintjes, Martha Schattenhofer, Andreas Ellrott, Jörg Wulf, Michael Zeder, M. Fuchs, M. Hecker, J. Peplies, F. D. Bockelmann, U. Callies, G. Gerdts, A. Wichels, K. H. Wiltshire, F. O. Glöckner, T. Schweder, and R. Amann. 2016. “Modification of a High-Throughput Automatic Microbial Cell.” Applied and Environmental Microbiology 82(11):3289–96. doi: 10.1128/AEM.03931-15.Editor.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 365 nm, 470 nm, 590 nm - Bosch, Daniel, Douglas Asede, and Ingrid Ehrlich. 2016. “Ex Vivo Optogenetic Dissection of Fear Circuits in Brain Slices.” Journal of Visualized Experiments 2016(110). doi: 10.3791/53628.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Darehgazani, Reyhaneh, Maryam Peymani, Motahare-Sadat Hashemi, Mir Davood Omrani, Abolfazl Movafagh, Kamran Ghaedi, and Mohammad Hossein Nasr-Esfahani. 2016. “PPARγ Ameliorated LPS Induced Inflammation of HEK Cell Line Expressing Both Human Toll-like Receptor 4 (TLR4) and MD2.” Cytotechnology 68(4):1337–48. doi: 10.1007/s10616-015-9893-6.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Füzesi, Tamás, Nuria Daviu, Jaclyn I. Wamsteeker Cusulin, Robert P. Bonin, and Jaideep S. Bains. 2016. “Hypothalamic CRH Neurons Orchestrate Complex Behaviours after Stress.” Nature Communications 7(1):11937. doi: 10.1038/ncomms11937.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2)
2015
- Agsten, Marianne, Sabine Hessler, Sandra Lehnert, Tilmann Volk, Andrea Rittger, Stephanie Hartmann, Christian Raab, Doo Yeon Kim, Teja W. Groemer, Michael Schwake, Christian Alzheimer, and Tobias Huth. 2015. “BACE1 Modulates Gating of KCNQ1 (Kv7.1) and Cardiac Delayed Rectifier KCNQ1/KCNE1 (IKs).” Journal of Molecular and Cellular Cardiology 89:335–48. doi: 10.1016/j.yjmcc.2015.10.006.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Hessler, Sabine, Fang Zheng, Stephanie Hartmann, Andrea Rittger, Sandra Lehnert, Meike Völkel, Matthias Nissen, Elke Edelmann, Paul Saftig, Michael Schwake, Tobias Huth, and Christian Alzheimer. 2015 “β-Secretase BACE1 Regulates Hippocampal and Reconstituted M-Currents in a β-Subunit-like Fashion.” Journal of Neuroscience 35(8):3298–3311. doi: 10.1523/JNEUROSCI.3127-14.2015.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Meyer, Arne, Christian Betzel, and Marc Pusey. 2015. “Latest Methods of Fluorescence-Based Protein Crystal Identification.” Acta Crystallographica Section F Structural Biology Communications 71(2):121–31. doi: 10.1107/S2053230X15000114.
Equipment used: UV-illumination Filter - Thoke, Henrik Seir, Asger Tobiesen, Jonathan Brewer, Per Lyngs Hansen, Roberto P. Stock, Lars F. Olsen, and Luis A. Bagatolli. 2015 “Tight Coupling of Metabolic Oscillations and Intracellular Water Dynamics in Saccharomyces Cerevisiae” edited by M. Polymenis. PLOS ONE 10(2):e0117308. doi: 10.1371/journal.pone.0117308.
Equipment used: from illumination systems a beam combination cube (FC-70 Series) and light guids - Wabnig, Sebastian, Jana Fiona Liewald, Szi Chieh Yu, and Alexander Gottschalk. 2015. “High-Throughput All-Optical Analysis of Synaptic Transmission and Synaptic Vesicle Recycling in Caenorhabditis Elegans.” PLoS ONE 10(8):1–26. doi: 10.1371/journal.pone.0135584.
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) with 470 and 590 nm
2014
- Binkert, Melanie, László Kozma-Bognár, Kata Terecskei, Lieven De Veylder, Ferenc Nagy, and Roman Ulm. 2014. “UV-B-Responsive Association of the Arabidopsis BZIP Transcription Factor ELONGATED HYPOCOTYL5 with Target Genes, Including Its Own Promoter.” The Plant Cell 26(10):4200–4213. doi: 10.1105/tpc.114.130716.
Equipment used: UV-illumination Filter - Costa, Wagner Steuer, Jana Liewald, and Alexander Gottschalk. 2014. “Photoactivated Adenylyl Cyclases as Optogenetic Modulators of Neuronal Activity.” Pp. 161–75 in Photoswitching Proteins: Methods and Protocols, edited by S. Cambridge. New York, NY: Springer New York.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Masson, Jean Baptiste, Patrice Dionne, Charlotte Salvatico, Marianne Renner, Christian G. Specht, Antoine Triller, and Maxime Dahan. 2014. “Mapping the Energy and Diffusion Landscapes of Membrane Proteins at the Cell Surface Using High-Density Single-Molecule Imaging and Bayesian Inference: Application to the Multiscale Dynamics of Glycine Receptors in the Neuronal Membrane.” Biophysical Journal 106(1):74–83. doi: 10.1016/j.bpj.2013.10.027.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Riol-Blanco, Lorena, Jose Ordovas-Montanes, Mario Perro, Elena Naval, Aude Thiriot, David Alvarez, Silke Paust, John N. Wood, and Ulrich H. Von Andrian. 2014. “Nociceptive Sensory Neurons Drive Interleukin-23-Mediated Psoriasiform Skin Inflammation.” Nature 510(7503):157–61. doi: 10.1038/nature13199.
Equipment used: Flash lamp system SP-20
2013
- Rahman, Jamilur, A. Tobias Latal, Stefanie Besser, Johannes Hirrlinger, and Swen Hülsmann. 2013 “Mixed Miniature Postsynaptic Currents Resulting from Co-Release of Glycine and GABA Recorded from Glycinergic Neurons in the Neonatal Respiratory Network.” European Journal of Neuroscience 37(8):1229–41. doi: 10.1111/ejn.12136.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 488 nm - Sachse, Carolyn C., Young Hye Kim, Marianne Agsten, Tobias Huth, Christian Alzheimer, Dora M. Kovacs, and Doo Yeon Kim. 2013. “BACE1 and Presenilin/γ-Secretase Regulate Proteolytic Processing of KCNE1 and 2, Auxiliary Subunits of Voltage-Gated Potassium Channels.” FASEB Journal 27(6):2458–67. doi: 10.1096/fj.12-214056.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Savery, Michele D., John X. Jiang, Pyong Woo Park, and Edward R. Damiano. 2013. “The Endothelial Glycocalyx in Syndecan-1 Deficient Mice.” Microvascular Research 87(1):83–91. doi: 10.1016/j.mvr.2013.02.001.
Equipment used: Double pulse flash lamp system DPS-1 - Vetter, Irina, Alexander Hein, Simon Sattler, Sabine Hessler, Filip Touska, Elisangela Bressan, Andres Parra, Ulrich Hager, Andreas Leffler, Stepana Boukalova, Matthias Nissen, Richard J. Lewis, Carlos Belmonte, Christian Alzheimer, Tobias Huth, Viktorie Vlachova, Peter W. Reeh, and Katharina Zimmermann. 2013. “Amplified Cold Transduction in Native Nociceptors by M-Channel Inhibition.” Journal of Neuroscience 33(42):16627–41. doi: 10.1523/JNEUROSCI.1473-13.2013.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2)
2012
- Goodman, Miriam B., Theodore H. Lindsay, Shawn R. Lockery, and Janet E. Richmond. 2012. “Electrophysiological Methods for Caenorhabditis Elegans Neurobiology.” Pp. 409–36 in Methods Cell biol. 107.
Equipment used: Dual single wavelength LED Illumination system KSL-2 (KSL-2) with 470 and 530 nm - Husson, Steven J., Jana F. Liewald, Christian Schultheis, Jeffrey N. Stirman, Hang Lu, and Alexander Gottschalk. 2012. “Microbial Light-Activatable Proton Pumps as Neuronal Inhibitors to Functionally Dissect Neuronal Networks in C. Elegans.” PLoS ONE 7(7). doi: 10.1371/journal.pone.0040937.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm - Petty, Howard R., and Andrea Clark. 2012. “Methods to Improve the Kinetic Evaluation of Fluorescence Intensities and Locations of Chemical Signals within Single Living Cells.” Pp. 1–189 in Proceedings of The Lille Spring School on, edited by P. Amar, F. E. Es, and V. Norris.
Equipment used: from illumination systems a beam combination cube (FC-70 Series) - Reichinnek, Susanne, Alexandra von Kameke, Anna M. Hagenston, Eckehard Freitag, Fabian C. Roth, Hilmar Bading, Mazahir T. Hasan, Andreas Draguhn, and Martin Both. 2012. “Reliable Optical Detection of Coherent Neuronal Activity in Fast Oscillating Networks in Vitro.” NeuroImage 60(1):139–52. doi: 10.1016/j.neuroimage.2011.12.018.
Equipment used: single wavelength LED Illumination system KSL-2 (KSL-2) with 470 nm
2011
- Fehér, Balázs, Lászlõ Kozma-Bognár, Éva Kevei, Anita Hajdu, Melanie Binkert, Seth Jon Davis, Eberhard Schäfer, Roman Ulm, and Ferenc Nagy. 2011. “Functional Interaction of the Circadian Clock and UV RESISTANCE LOCUS 8-Controlled UV-B Signaling Pathways in Arabidopsis Thaliana.” Plant Journal 67(1):37–48. doi: 10.1111/j.1365-313X.2011.04573.x.
Equipment used: UV-illumination Filter - Habibagahi, Arezoo, Youssef Mébarki, Yasir Sultan, and Robert J. Crutchley. 2011. “Luminophore Charge Effects in Water-Based Oxygen Sensor Films.” Journal of Photochemistry and Photobiology A: Chemistry 225(1):88–94. doi: 10.1016/j.jphotochem.2011.10.003.
Equipment used: UV-illumination Filter - Maiguel, Dony, Mohd Hafeez Faridi, Changli Wei, Yoshihiro Kuwano, Keir M. Balla, Dayami Hernandez, Constantinos J. Barth, Geanncarlo Lugo, Mary Donnelly, Ali Nayer, Luis F. Moita, Stephan Schürer, David Traver, Phillip Ruiz, Roberto I. Vazquez-Padron, Klaus Ley, Jochen Reiser, and Vineet Gupta. 2011. “Small Molecule–Mediated Activation of the Integrin CD11b/CD18 Reduces Inflammatory Disease.” Science Signaling 4(189):ra57–ra57. doi: 10.1126/scisignal.2001811.
Equipment used: Double pulse flash lamp system DPS-1
2010
- Chiaruttini, N., M. de Frutos, E. Augarde, P. Boulanger, L. Letellier, and V. Viasnoff. 2010. “Is the In Vitro Ejection of Bacteriophage DNA Quasistatic? A Bulk to Single Virus Study.” Biophysical Journal 99(2):447–55. doi: 10.1016/j.bpj.2010.04.048.
Equipment used: High power short wave UV illumination system UVICO (UVICO-2) - Clark, Andrea J., Roberto Romero, and Howard R. Petty. 2010. “Improved Detection of Nicotinamide Adenine Dinucleotide Phosphate Oscillations within Human Neutrophils.” Cytometry Part A 77(10):976–82. doi: 10.1002/cyto.a.20961.
Equipment used: Spot illumination module (AiWon) with UV LED System UVILED - Clark, Andrea J., Michelle Diamond, Megan Elfline, and Howard R. Petty. 2010. “Calicum Microdomains Form within Neutrophils at the Neutrophil–Tumor Cell Synapse: Role in Antibody-Dependent Target Cell Apoptosis.” Cancer Immunology, Immunotherapy 59(1):149–59. doi: 10.1007/s00262-009-0735-2.
Equipment used: from illumination systems a beam combination cube (FC-70 Series) - Elfline, Megan, Andrea Clark, Howard R. Petty, and Roberto Romero. 2010. “Bi-Directional Calcium Signaling Between Adjacent Leukocytes and Trophoblast-Like Cells.” American Journal of Reproductive Immunology 23(1):1–7. doi: 10.1111/j.1600-0897.2010.00839.x.
Equipment used: from illumination systems a beam combination cube (FC-70 Series) - Olsen, Lars Folke, Ann Zahle Andersen, Anita Lunding, Jens Christian Brasen, and Allan K. Poulsen. 2009. “Regulation of Glycolytic Oscillations by Mitochondrial and Plasma Membrane H+-ATPases.” Biophysical Journal 96(9):3850–61. doi: 10.1016/j.bpj.2009.02.026.
Equipment used: from illumination systems a beam combination cube (FC-70 Series)
2009
- Andersen, Ann Zahle, Ana Lúcia Carvalho, Ana Rute Neves, Helena Santos, Ursula Kummer, and Lars Folke Olsen. 2009. “The Metabolic PH Response in Lactococcus Lactis: An Integrative Experimental and Modelling Approach.” Computational Biology and Chemistry 33(1):71–83. doi: 10.1016/j.compbiolchem.2008.08.001.
Equipment used: from illumination systems, liquid light guide - Olsen, Lars Folke, Ann Zahle Andersen, Anita Lunding, Jens Christian Brasen, and Allan K. Poulsen. 2009. “Regulation of Glycolytic Oscillations by Mitochondrial and Plasma Membrane H+-ATPases.” Biophysical Journal 96(9):3850–61. doi: 10.1016/j.bpj.2009.02.026.
Equipment used: from illumination systems, liquid light guide - Pickard, John E., and Klaus Ley. 2009. “Micro-PTV Measurement of the Fluid Shear Stress Acting on Adherent Leukocytes in Vivo.” Biophysical Journal 96(10):4249–59. doi: 10.1016/j.bpj.2009.01.060.
Equipment used: Double pulse flash lamp system DPS-1
2008
- Cipriano, Bani H., Arun K. Kota, Alan L. Gershon, Conrad J. Laskowski, Takashi Kashiwagi, Hugh A. Bruck, and Srinivasa R. Raghavan. 2008. “Conductivity Enhancement of Carbon Nanotube and Nanofiber-Based Polymer Nanocomposites by Melt Annealing.” Polymer 49(22):4846–51. doi: 10.1016/j.polymer.2008.08.057.
Equipment used: UV-illumination Filter - Dona, Maria, Gabrielle Fredman, Jan M. Schwab, Nan Chiang, Makoto Arita, Ahmad Goodarzi, Guiying Cheng, Ulrich H. von Andrian, and Charles N. Serhan. 2008. “Resolvin E1, an EPA-Derived Mediator in Whole Blood, Selectively Counterregulates Leukocytes and Platelets.” Blood 112(3):848–55. doi: 10.1182/blood-2007-11-122598.
Equipment used: Flash lamp system SP-20 - Imai, Yoichi, Eun Jeong Park, Dan Peer, António Peixoto, Guiying Cheng, Ulrich H. von Andrian, Christopher V. Carman, and Motomu Shimaoka. 2008“Genetic Perturbation of the Putative Cytoplasmic Membrane-Proximal Salt Bridge Aberrantly Activates Α4 Integrins.” Blood 112(13):5007–15. doi: 10.1182/blood-2008-03-144543.
Equipment used: Flash lamp system SP-20 - Massaro, Sebastiano, Theodora Zlateva, Vincent Torre, and Luca Quaroni. 2008. “Detection of Molecular Processes in the Intact Retina by ATR-FTIR Spectromicroscopy.” Analytical and Bioanalytical Chemistry 390(1):317–22. doi: 10.1007/s00216-007-1710-4.
Equipment used: high power short wave UV illumination system UVICO (UVICO-2) - Popp, David, Akihiro Yamamoto, Mitsusada Iwasa, Yasushi Nitanai, and Yuichiro Maéda. 2008 “Single Molecule Polymerization, Annealing and Bundling Dynamics of SipA Induced Actin Filaments.” Cell Motility and the Cytoskeleton 65(2):165–77. doi: 10.1002/cm.20252.
Equipment used: Photomanipulation system AiWon (AiWon-Series) - Potter, Daniel R., and Edward R. Damiano. 2008. “The Hydrodynamically Relevant Endothelial Cell Glycocalyx Observed in Vivo Is Absent in Vitro.” Circulation Research 102(7):770–76. doi: 10.1161/CIRCRESAHA.107.160226.
Equipment used: Double pulse flash lamp system DPS-1 - Savery, Michele D., and Edward R. Damiano. 2008. “The Endothelial Glycocalyx Is Hydrodynamically Relevant in Arterioles throughout the Cardiac Cycle.” Biophysical Journal 95(3):1439–47. doi: 10.1529/biophysj.108.128975.
Equipment used: stroboscopic double pulse flash lamp system DPS-1
2007
- Cipiriano, Bani H., Takashi Kashiwagi, Srinivasa R. Raghavan, Ying Yang, Eric A. Grulke, Kazuya Yamamoto, John R. Shields, and Jack F. Douglas. 2007. “Effects of Aspect Ratio of MWNT on the Flammability Properties of Polymer Nanocomposites.” Polymer 48(20):6086–96. doi: 10.1016/j.polymer.2007.07.070.
Equipment used: UV-illumination Filter - Clark, Andrea J., and Howard R. Petty. 2007. “Super-Quiet Microfluorometry : Examples of Tumor Cell Metabolic Dynamics.” Pp. 403–8 in Modern Research and Educational Topics in Microscopy, edited by J. A. Méndez-Vilas and J. Díaz.
Equipment used: from illumination systems a beam combination cube (FC-70 Series) - Kashiwagi, Takashi, Jeffrey Fagan, Jack F. Douglas, Kazuya Yamamoto, Alan N. Heckert, Stefan D. Leigh, Jan Obrzut, Fangming Du, Sheng Lin-Gibson, Minfang Mu, Karen I. Winey, and Reto Haggenmueller. 2007. “Relationship between Dispersion Metric and Properties of PMMA/SWNT Nanocomposites.” Polymer 48(16):4855–66. doi: 10.1016/j.polymer.2007.06.015.
Equipment used: UV-illumination Filter - Popp, David, Akihiro Yamamoto, and Yuichiro Maéda. 2007. “Crowded Surfaces Change Annealing Dynamics of Actin Filaments.” Journal of Molecular Biology 368(2):365–74. doi: 10.1016/j.jmb.2007.01.087.
Equipment used: Photomanipulation system AiWon (AiWon-Series) - Wood, Christopher D., Takuya Nishigaki, Yoshiro Tatsu, Noboru Yumoto, Shoji A. Baba, Michael Whitaker, and Alberto Darszon. 2007. “Altering the Speract-Induced Ion Permeability Changes That Generate Flagellar Ca2+ Spikes Regulates Their Kinetics and Sea Urchin Sperm Motility.” Developmental Biology 306(2):525–37. doi: 10.1016/j.ydbio.2007.03.036.
Equipment used: from illumination systems a beam combination cube (FC-70 Series)
2006
- Herpens, A., S. Schagen, S. Scheede, and Boris Kristof. 2006 “Evaluation of Comedogenic Activity by Skin Fluorescence Imaging Analysis (Skin Analyzing Fluorescence Imaging Recorder).” in Bioengineering of the Skin: Skin Imaging & Analysis, edited by K.-P. Wilhelm, P. Elsner, E. Berardesca, and H. I. Maibach.
Equipment used: flash lamp system - Neuert, Gregor, Thorsten Hugel, Roland R. Netz, and Hermann E. Gaub. 2006. “Elasticity of Poly(Azobenzene−peptides).” Macromolecules 39(2):789–97. doi: 10.1021/ma051622d.
Equipment used: flash lamp system - Nishigaki, Takuya, Christopher D. Wood, Kogiku Shiba, Shoji A. Baba, and Alberto Darszon. 2006. “Stroboscopic Illumination Using Light-Emitting Diodes Reduces Phototoxicity in Fluorescence Cell Imaging.” BioTechniques 41(2):191–97. doi: 10.2144/000112220.
Equipment used: from illumination systems a beam combination cube (FC-70 Series) - Sperandio, Markus, John Pickard, Sunil Unnikrishnan, Scott T. Acton, and Klaus Ley. 2006. “Analysis of Leukocyte Rolling In Vivo and In Vitro.” Pp. 346–71 in Methods in enzymology. 416.
Equipment used: Double pulse flash lamp system DPS-1
2004
- Charbonnière, Loïc J., Nicolas Weibel, Claude Estournes, Cedric Leuvrey, and Raymond Ziessel. 2004. “Spatial and Temporal Discrimination of Silica Particles Functionalised with Luminescent Lanthanide Markers Using Time-Resolved Luminescence Microscopy.” New J. Chem. 28(7):777–81. doi: 10.1039/B402024C.
Equipment used: Flash lamp system JML-C2 - Ritter, Eglof, Kerstin Zimmermann, Martin Heck, Klaus Peter Hofmann, and Franz J. Bartl. 2004 “Transition of Rhodopsin into the Active Metarhodopsin II State Opens a New Light-Induced Pathway Linked to Schiff Base Isomerization.” Journal of Biological Chemistry 279(46):48102–11. doi: 10.1074/jbc.M406857200.
Equipment used: Flash lamp system JML-C2
FLUCS Micro Flow
2023
- Ng, KangBo, Nisha Hirani, Tom Bland, Joana Borrego-Pinto, Susan Wagner, Moritz Kreysing, and Nathan W. Goehring. “Cleavage Furrow-Directed Cortical Flows Bias PAR Polarization Pathways to Link Cell Polarity to Cell Division.” Current Biology 33(20):4298-4311.e6. doi: 10.1016/j.cub.2023.08.076.
Equipment used: Micro Flow photomanipulation system FLUCS
2022
- Stoev, Ilya D., Florian Huhn, Nicola Maghelli, Elena Erben, Sebastian Bundschuh, Benjamin Seelbinder, Catarina Nabais, Britta Schroth-Diez, Ines Höfer, Jan Peychl, Moritz Kreysing, and Gert Rapp. 2022. “FLUCS – Focused Light-Induced Cytoplasmic Streaming.” Wiley Analytical Science. https://analyticalscience.wiley.com/do/10.1002/was.0004000255
Equipment used: Micro Flow photomanipulation system FLUCS