Mouse embryonic fibroblasts (MEF) were transfected with different mutations of the adaptor protein vinculin and FRAP experiments were performed to analyze the influence of the mutations on the incorporation of vinculin into focal adhesions (FAs).
For the quantification of the real protein exchange dynamics within focal adhesions without overlying cytosolic diffusion artifacts, the FRAP technique was combined with TIRF (total internal reflection fluorescence) microscopy. To this end, a Zeiss Observer Z.1 (TIRF) microscope was equipped with a Rapp OptoElectronic fixed spot illumination device coupled to a 473 nm diode laser (DL-473). In combination with a 100x objective, a spot size of approximately 5 µm in diameter was illuminated, allowing bleaching of single focal adhesions in living cells. The results indicate a slight, but significant increase in mobile fraction for the Y1065E-mutant and an uncoordinated incorporation of the Y1065F-mutant into FAs.
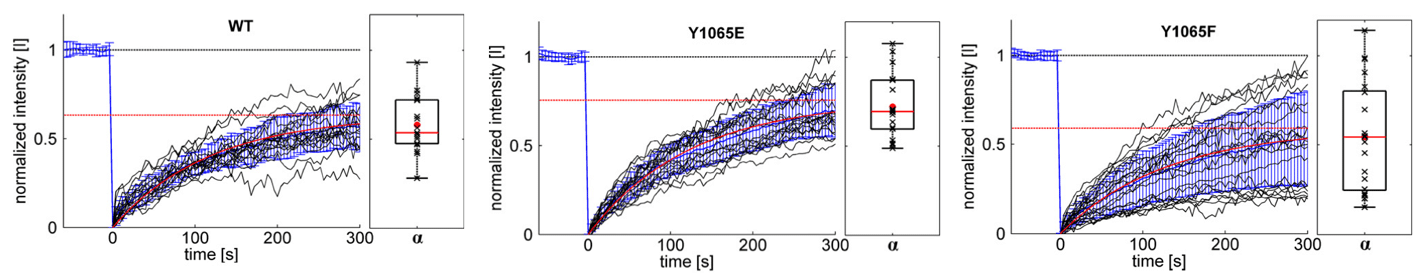
Figure 1: Exchange dynamics of different vinculin constructs measured with a TIRF-FRAP setup. Different eGFP-vinculin constructs (WT, Y1065E and Y1065F) were expressed in vin‑/‑ MEFs. Before bleaching, cells were monitored for at least 5 minutes to ensure that only stable FAs were analyzed. Black graphs show the normalized intensity [I] curves from individual measurements. The mean value and its standard deviation σ are shown in blue; results from the fit of mean values to the kinetic model are shown in red. The saturation value α indicates the mobile fraction (red dotted line). At the right of each graph, the box plots show the distribution of the mobile fractions of single measurements (black “x”). The mean values are given as a red dot, the median as a red line.
Setup:
- Microscope: Zeiss Observer Z.1 with TIRF
- Objective: 100x α-Plan-Apochromat (oil) NA 1.46
Rapp OptoElectronic components:
- FRAP System: ZSI fixed spot illumination
- Light source: DL-473 diode laser
- Optical fiber: multimode (ø = 550 µm) => spot size on sample approx. 5 µm
Published:
Küpper at al. 2010. “Tyrosine Phosphorylation of Vinculin at Position 1065 Modifies Focal Adhesion Dynamics and Cell Tractions.” Biochemical and Biophysical Research Communications 399 (4): 560–64. doi:10.1016/j.bbrc.2010.07.110.


