Laser Photolysis of Caged Calcium (NP-EGTA)
Hippocampal slices of P16 mice were stained with the cell-permeant calcium-sensitive dye Fluo-4 AM and additionally loaded with
cell permeant NP-caged EGTA AM. This photolabile chelator releases Ca2+ upon UV illumination due to its Kd increase from 80 nM to >1 mM. Uncaging of calcium was achieved by the application of brief (500 ms) and focally restricted flashes of UV light (diameter ~1 μm) to astrocytic somata during two-photon-imaging of these cells. The uncaging unit consisted of a DL-355/10 UV-laser, operated by a UGA-40 galvanometric controlling system (Rapp OptoElectronic). This unit was coupled to a custom-built multiphoton laser scanning microscope (based on a Fluoview 300, Olympus; 60x/1.1, NIR Apo, Nikon, water immersion objective), connected to a tuneable Ti:Sa laser (MaiTai, SpectraPhysics).
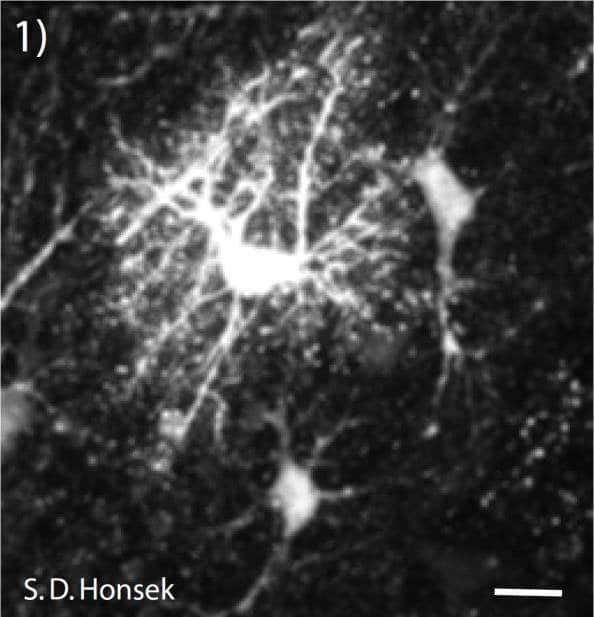
Figure 1: Multi-photon-image of astrocytes in the stratum radiatum of a mouse hippocampus. To specifically identify astrocytes in the hippocampal slice preparation, slices were loaded with the astocyte specific vital dye sulforhodamine 101 (SR 101). Because Fluo-4 fluorescence is very dim at low intracellular baseline calcium levels, a single astrocyte was additionally filled with AlexaFluor 594 to visualize its fine processes. (Scale bar = 60 μm)
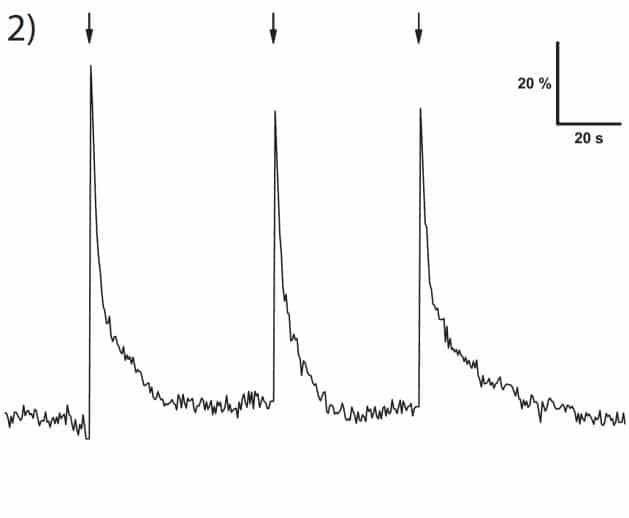
Figure 2: Calcium transients as indicated by changes in fluorescence (ΔF/F0). Uncaging UV flashes (500 ms) were aimed at the soma of the astrocyte.
Setup:
- Microscope: Olympus Fluoview 300
- Objective: Nikon 60x NIR Apo NA 1.1 water immersion
Rapp OptoElectronic components:
- System: UGA-40 – point scanning device
- Light source: DL-355/10 diode laser
- Approx. sample spot size: 1 μm
Data by courtesy of:
J. Langer, C. Kleinhans & C.R. Rose
Institute for Neurobiology (Heinrich-Heine-University)
Düsseldorf (Germany)